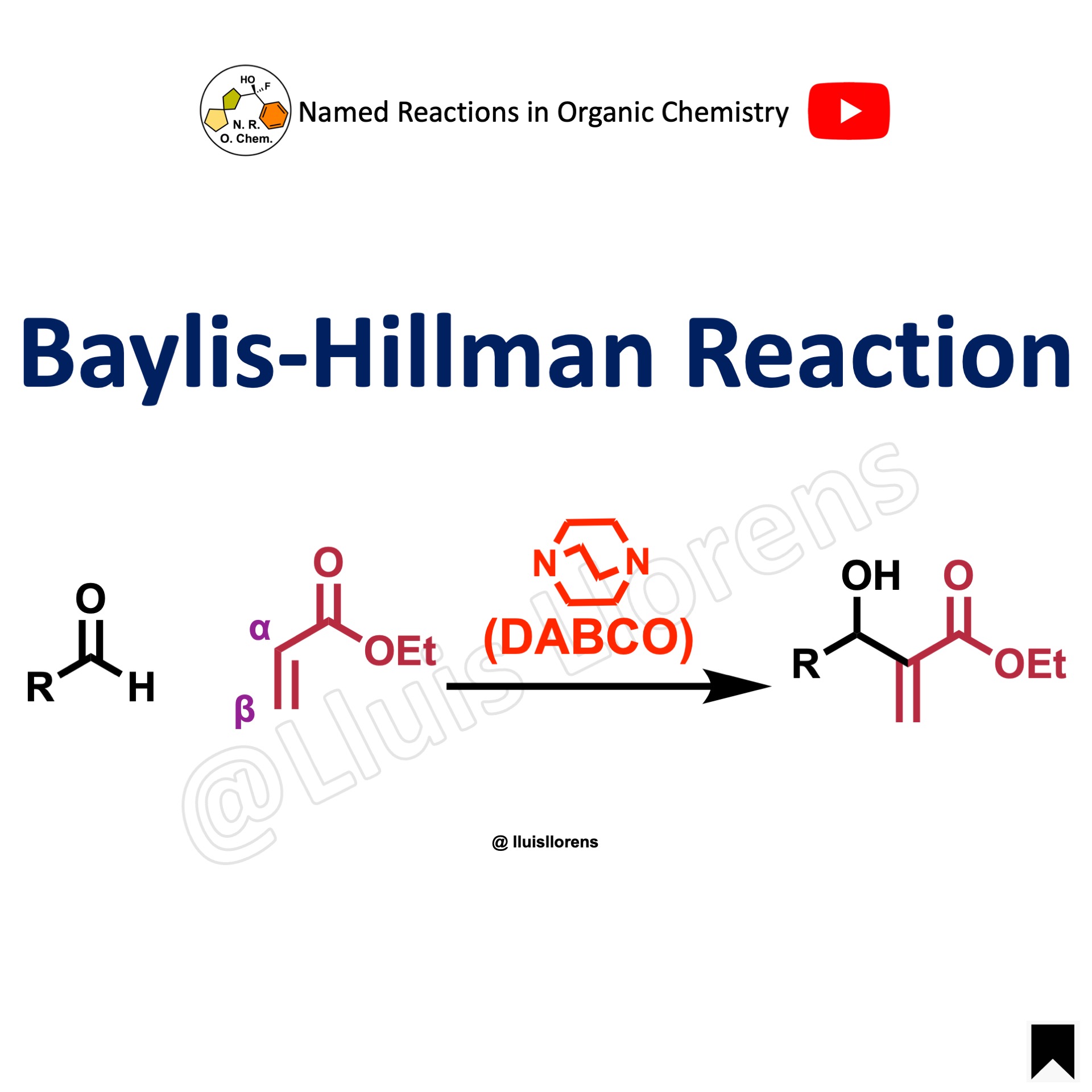
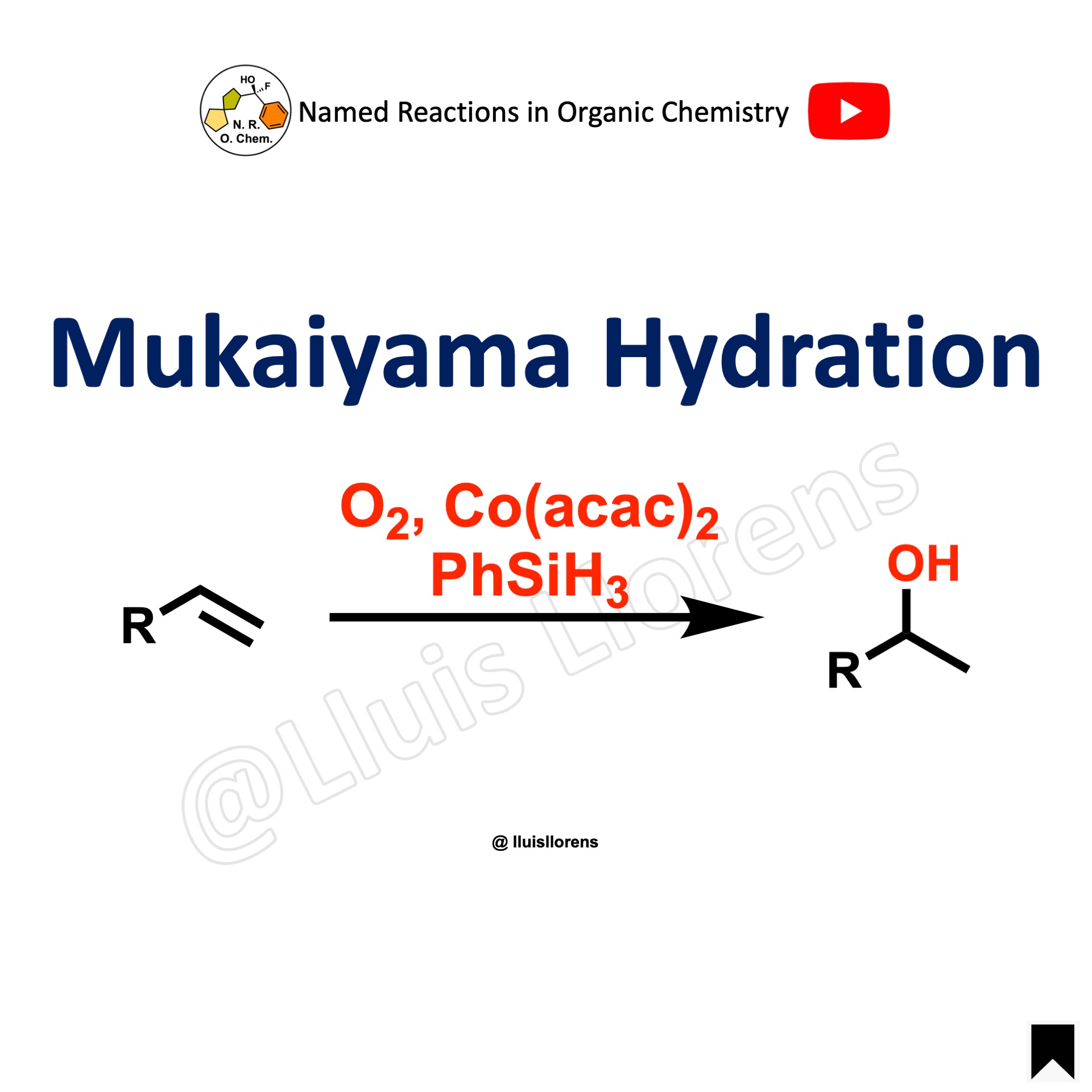
The Michael reaction or Michael addition is a C–C bond-forming reaction. It consists of the nucleophilic addition of an activated nucleophile (Michael donor) to an α,β-unsaturated carbonyl compound (Michael acceptor). It belongs to the class of conjugate additions or 1,4-addition reactions.

- The main drawback of the Michael reaction is that other processes may compete with the desired 1,4-addition. For example 1,2-addition reactions or self-condensation of the carbon nucleophile. These side reactions can be suppressed or minimized by the use of certain additives or solvents.
- Acid-catalyzed Michael reaction, see example 1.
- Base-catalyzed Michael reaction, see example 2.
- Oxa-Michael reaction, see example 3.
- Aza-Michael reaction, see example 4.
- Thia-Michael reaction, see example 5.
Reaction mechanism of Michael reaction
(i) Deprotonation of the Michael donor by a base. (ii) Conjugate addition reaction between the nucleophile and the Michael acceptor. (iii) Proton abstraction from the protonated base leads to the final product.

Examples and experimental procedures of Michael reactions
Example 5: Angew. Chem. Int. Ed. 2024, 63, e202319070. Open access.

2-(Trimethylsilyl)ethanethiol (3.0 equiv) and DBU (2.4 equiv) were added at ambient temperature to a solution of the substrate (0.56 mmol, 1.0 equiv) in THF (2.2 mL). After stirring for 3 h, the mixture was diluted with hexanes (4.0 mL) and then filtered through a pad of SiO2, which was carefully rinsed with a mixture of tert-butyl methyl ether/hexanes (1:3). After removing the organic solvent, the resulting crude sulfide was used in the next step without further purification.
Example 4: J. Am. Chem. Soc. 2022, 144, 16042.

In a nitrogen filled glovebox were added the catalyst (0.001 equiv) and silver trifluoromethanesulfonate (0.0012 equiv) to a 25 mL vial charged with a magnetic stir bar. The vial was taken outside of the glovebox and THF (3 mL) and water (3 mL) were added. The mixture was stirred for 2 min, and the substrate (5.0 mmol, 1.0 equiv) was added. The reaction mixture was warmed up to 40 ºC and stirred for 12 h. The mixture was co-evaporated with toluene three times, and the residue was dissolved in DCM (25 mL). DBU (2.0 equiv) was added dropwise to this solution at room temperature and stirred for 15 h, quenched with H2O, and then extracted with DCM. The combined organic phase was washed with brine, dried over Na2SO4 and concentrated in vacuo. The residue was purified by flash column chromatography.
Example 3: J. Am. Chem. Soc. 2022, 144, 20539. Open access.

The crude substrate (0.01 mmol, 1.0 equiv) was dissolved in DCM (0.7 mL). Then, p-TsOH·H2O (1.0 equiv) was added in one portion, causing the color of the mixture to change from bright pink to colorless. The colorless solution was stirred for 1 h at room temperature. Following this period, the reaction was quenched with sat. aq. NaHCO3 and extracted with DCM. The combined organic layers were washed with brine, dried over MgSO4, filtered, and concentrated in vacuo to afford a pink residue. Flash column chromatography afforded the product.
Example 2: Angew. Chem. Int. Ed. 2020, 59, 22039.

The Michael acceptor, acrylonitrile (1.2 equiv), was added dropwise to a mixture of the racemic Michael donor (101.9 mmol, 1.0 equiv) and anhydrous K2CO3 (2.0 equiv) in acetone (500 mL) at room temperature. The reaction mixture was stirred for 40 h at the same temperature. After completion of the reaction, as monitored by TLC, the reaction mixture was filtered through a pad of Celite and concentrated under vacuum to remove the solvent. The resulting crude residue was diluted with EtOAc and washed with water and brine. The combined organic layers were dried over Na2SO4 and concentrated under vacuum. The crude compound was purified by flash column chromatography to deliver the racemic Michael adduct.
Example 1: J. Am. Chem. Soc. 2019, 141, 19589.

To a suspension of 2-methyl-1,3-cyclohexanedione (184 mmol, 1.0 equiv) in water (220 mL), freshly distilled acrolein (1.5 equiv) over anhydrous calcium sulfate was added. The reaction flask was wrapped in foil to exclude light. Acetic acid (0.05 equiv) was added dropwise at 23 ºC, and the reaction mixture was stirred vigorously. After 20 h, the aqueous reaction mixture was extracted with EtOAc. The combined organic layers were washed with brine, and the organic solution was dried over MgSO4. The dried solution was filtered, and the filtrate was concentrated to yield the aldehyde, which may be used without further purification.
Videos about Michael reaction
Images of Michael reaction
Online database of named reactions
Browse named reactions in alphabetical order or by category in our online database of organic reactions.