Mukaiyama Hydration
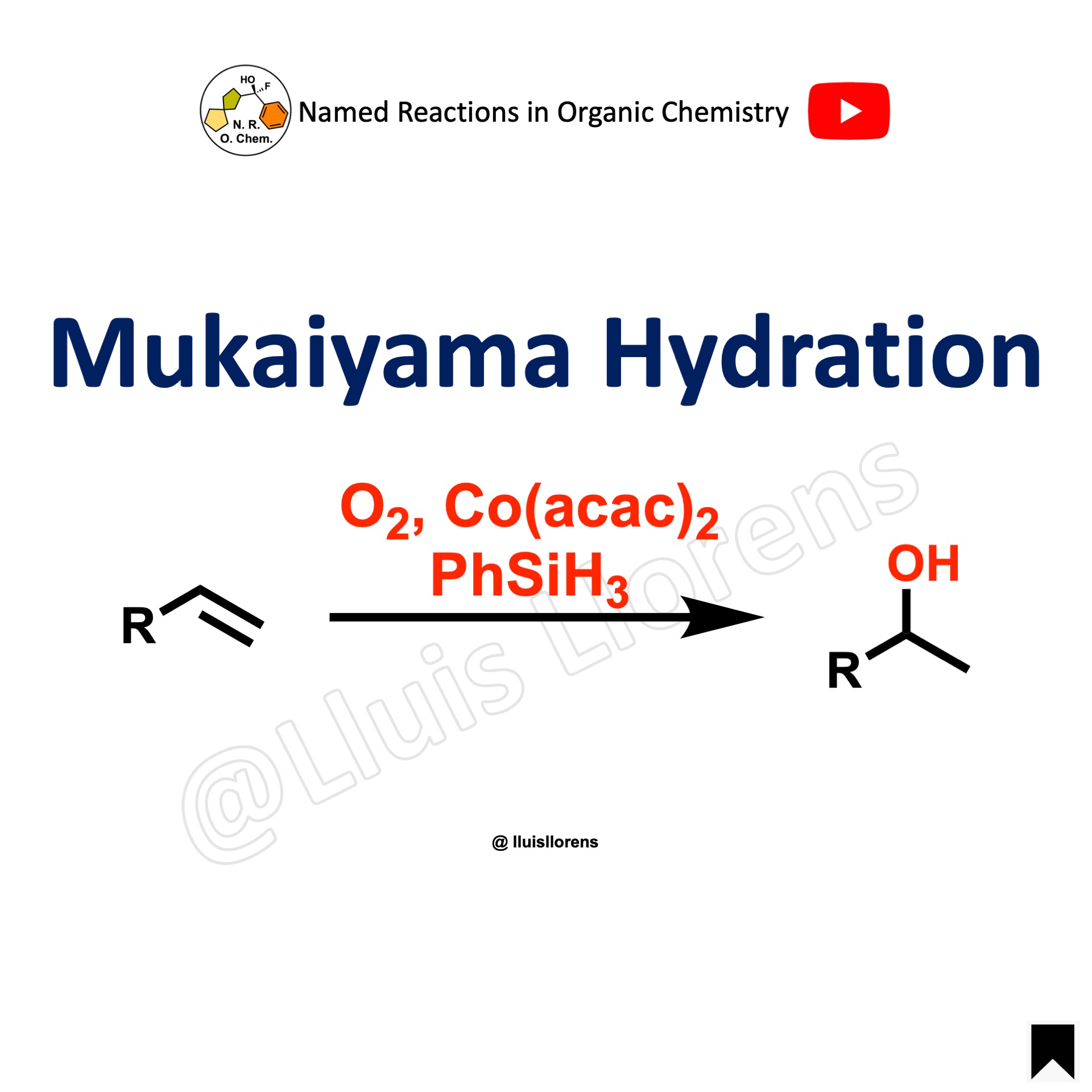
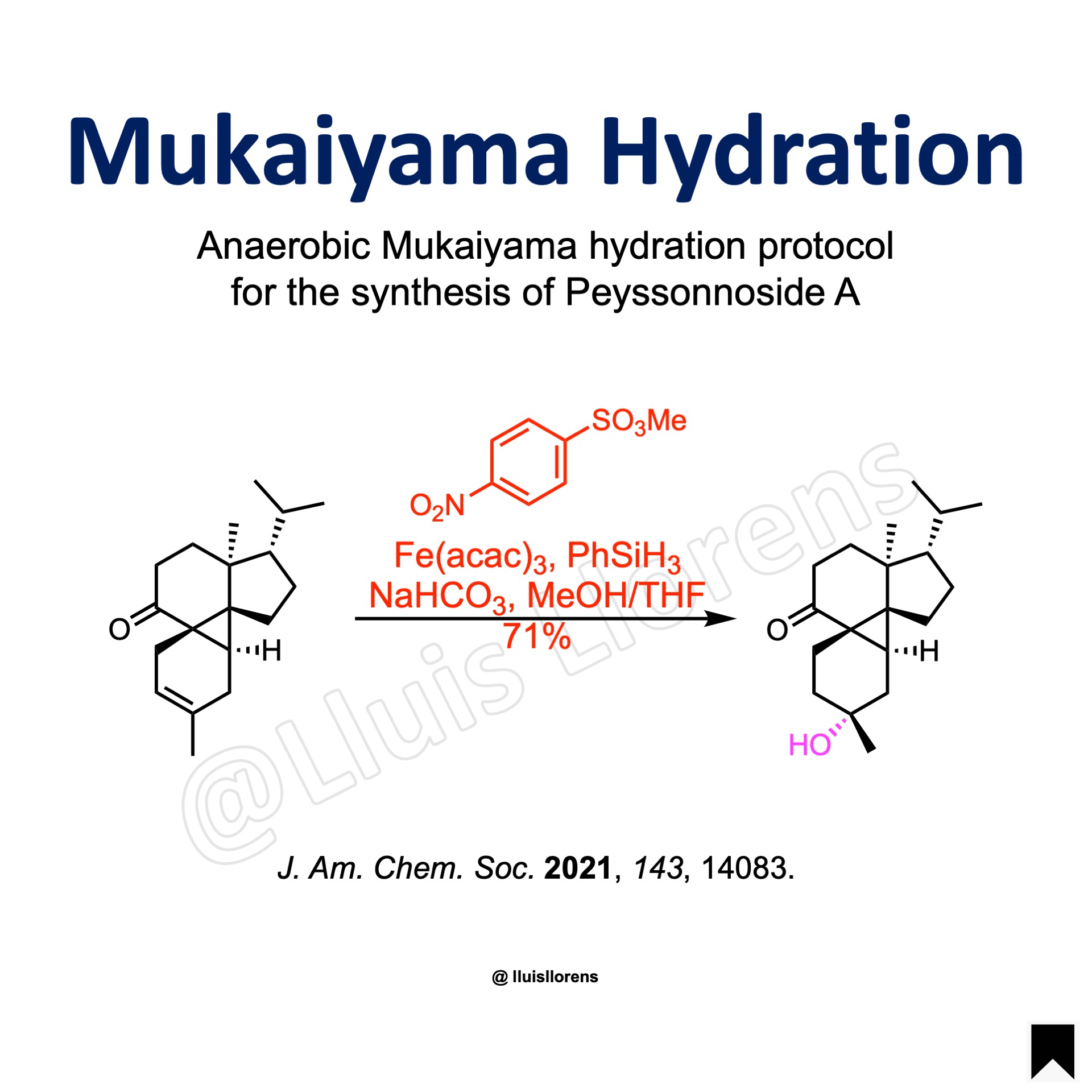
The Mukaiyama hydration allows the transformation of an olefin to the corresponding alcohol with Markovnikov selectivity.
General features: 1. The use of a silane reductant allows for this reaction to be carried out without heat. 2. The addition of t-butylhydroperoxide can increase the rate of slower-reacting substrates.
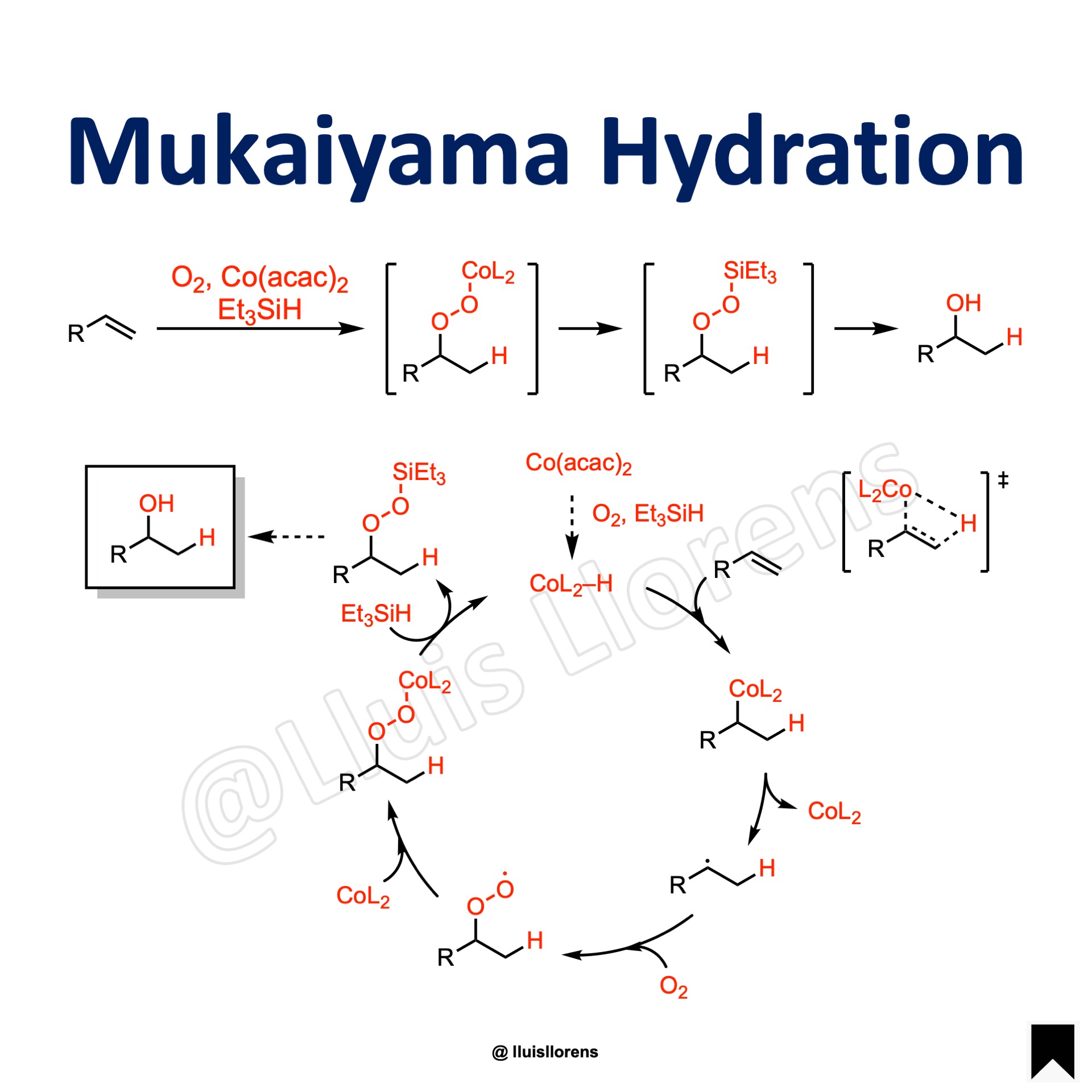
Reaction Mechanism
Formation of a metal hydride complex. Insertion of the double bond into the metal hydride generates a cobalt-alkyl bond that undergoes homolytic cleavage. The resulting carbon centered radical reacts with molecular oxygen, generating a chemical species that is subsequently trapped by the cobalt complex to form a cobalt-peroxide adduct. Final transmetalation with triethylsilane regenerates the metal hydride and delivers the desired product with Markovnikov selectivity upon further reduction of the silyl peroxide.
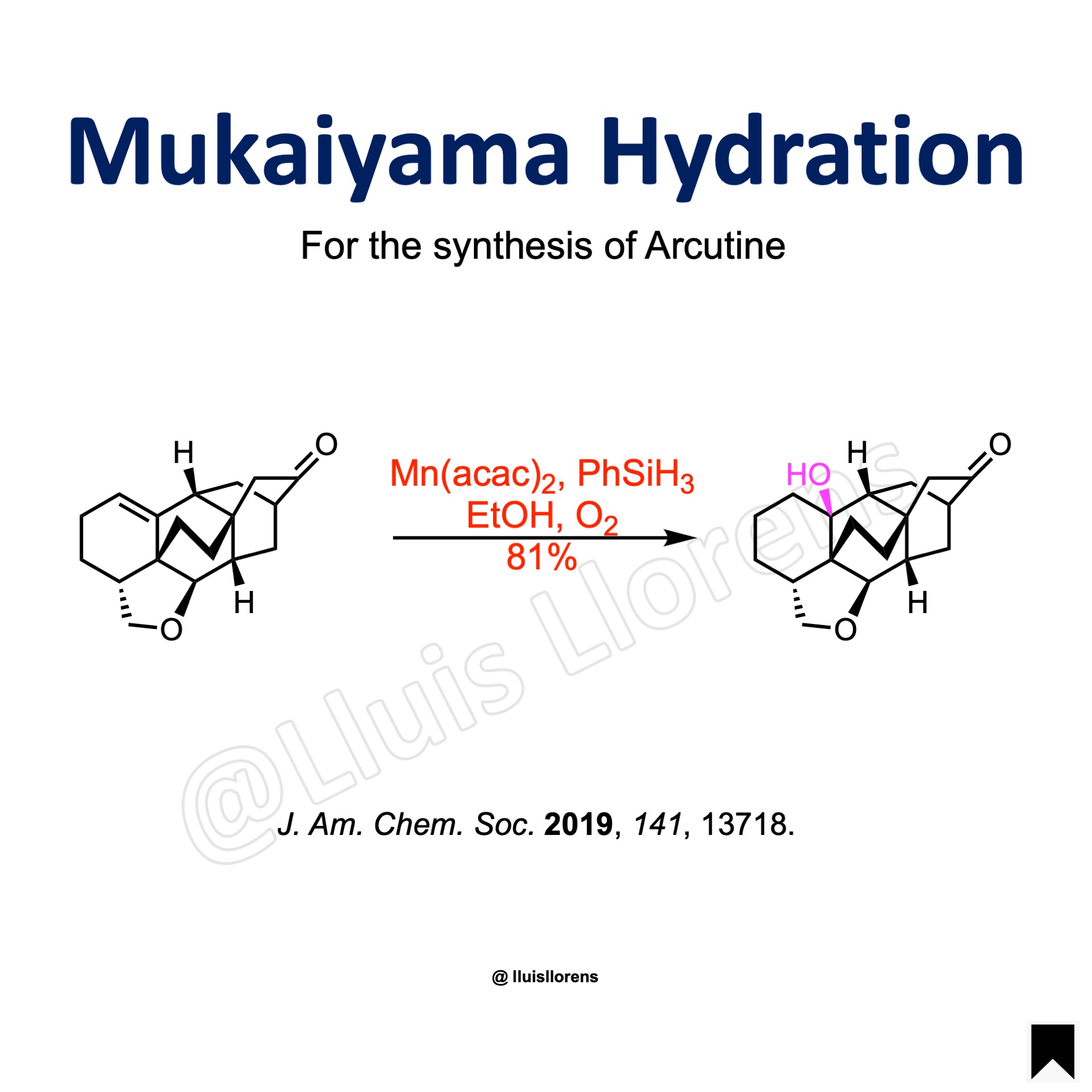
Examples
Experimental Procedure
To a stirred solution of the boronic ester (10.9 mmol, 1.0 eq) in CH2Cl2/MeOH (1:1, 109 mL) at room temperature was added 4Å molecular sieves (activated, 6.0 g), Cu(OAc)2 (1.0 eq) and DMAP (2.0 eq). The resulting mixture was stirred for 8 h before it was filtered through a pad of silica gel, eluted with EtOAc, and concentrated under reduced pressure. The resulting residue was purified by flash silica gel column chromatography to afford the desired product (60% yield).
Additional Examples
J. Am. Chem. Soc. 2021, 143, 14083. Open access.
Learn More Named Reactions