The Fischer esterification is a reaction between a carboxylic acid and an alcohol in the presence of an acid catalyst to produce the corresponding ester.
- The Fischer esterification is straightforward. Acidic conditions can be used if acid-sensitive functional groups are not an issue.
- The main by-product is water.
- Less environmental impact by reducing waste (atom economy) and the harmfulness of the reagents when compared to other procedures using acid chlorides, alkyl halides, or acid anhydrides.
- The reaction is a thermodynamically controlled process; the more stable ester tends to be the main product.
- The primary disadvantages of the Fischer esterification routes are its thermodynamic reversibility and relatively slow reaction rates.
Reaction mechanism of Fischer esterification
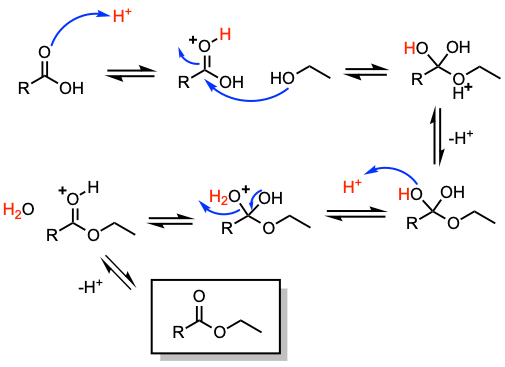
1. Proton transfer to the oxygen atom of the carbonyl group.
2. Nucleophilic addition to the carbonyl carbon.
3. Proton transfer.
4. Loss of water and formation of the ester.
Examples and experimental procedures of Fischer esterification
Example 2: ChemMedChem 2020, 15, 749.
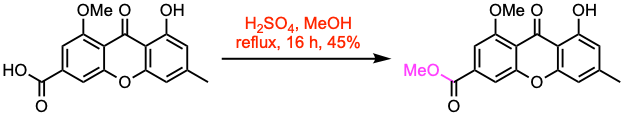
In a round-bottom flask, the carboxylic acid (466 μmol, 1.0 equiv) was dissolved in MeOH (10 mL), and H2SO4 (3.0 equiv) was added. The reaction mixture was heated under reflux overnight. Water and NaHCO3 were then added to the flask, and the solution was extracted with EtOAc. The organic layer was washed with brine, dried over Na2SO4, filtered, and the solvent was evaporated. The crude product was purified by crystallization with DCM and MeOH to afford the methyl ester.
Example 1: Chem. Ber. 1901, 34, 1457.

Videos about Fischer esterification
Images of Fischer esterification
Online database of named reactions
Browse named reactions in alphabetical order or by category in our online database of organic reactions.