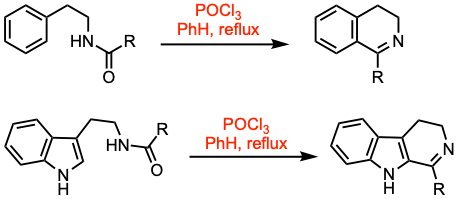
The Bischler-Napieralski reaction is an electrophilic aromatic substitution that facilitates the cyclization of β-arylethylamides or β-arylethylcarbamates using a condensing agent such as phosphorus oxychloride (POCl3), phosphorus pentoxide (P2O5), or zinc chloride (ZnCl2).
- The Bischler-Napieralski (B-N) reaction is carried out in refluxing acidic conditions and dehydrating agents such as POCl3 or Tf2O and polyphosphoric acid (Eaton’s reagent).
- The B-N reaction is one of the most frequently used methods for the synthesis of 3,4-dihydroisoquinoline derivatives.
- The Bischler-Napieralski reaction is most effective in the presence of electron-donating groups on the benzene ring.
- The products of the reaction can be easily dehydrogenated, being aromatized to the corresponding isoquinolines.
- In addition to the B-N reaction, another important method for the synthesis of the isoquinoline nucleus is the Pictet-Spengler reaction.
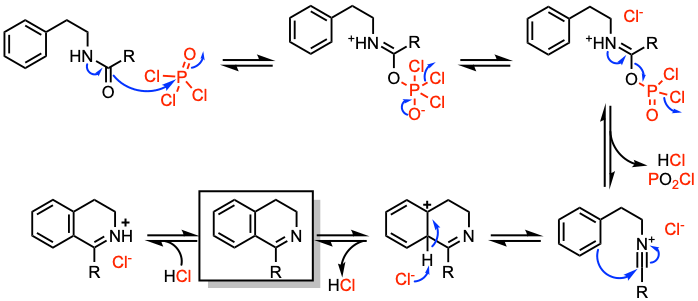
Reaction mechanism of Bischler-Napieralski reaction

The Bischler-Napieralski reaction presents two plausible mechanistic pathways:
1. Path (a) involves the generation of a dichlorophosphoryl imine-ester intermediate, which upon cyclization, leads to elimination with imine formation. Since the dehydroisoquinoline nitrogen is basic, neutralization is required to obtain the deprotonated product.
2. Path (b) includes the formation of a nitrilium ion intermediate followed by cyclization to dihydroisoquinoline. It is often assumed that different conditions affect the predominance of one mechanism over the other.
For further details on the Bischler-Napieralski reaction mechanism, refer to Adv. Heterocycl. Chem. 2014, 112, 183.
Examples and experimental procedures of Bischler-Napieralski reaction
Example 2: J. Am. Chem. Soc. 2023, 145, 20062.

To a solution of the amide (4.79 mmol, 1.0 equiv) in DCM (40 mL) at -20 ºC was added 2-chloropyridine (2.0 equiv), and the resulting mixture was allowed to stir at -20 ºC for 5 min before the addition of Tf2O (1.25 equiv). The resulting mixture was allowed to stir at -20 ºC for 30 min and then at 0 ºC for a further 20 min (solution color changed from yellow to dark red). A solution of NaBH4 (12 equiv) in MeOH (20 mL) was added at 0 ºC, and the resulting mixture was allowed to stir while slowly warming to 22 ºC over 1 h. The reaction was quenched by the addition of H2O and extracted with DCM. The combined organics were washed with brine, dried over MgSO4, filtered, and concentrated in vacuo. Purification by silica gel chromatography gave the tertiary amine.
Example 1: Org. Lett. 2020, 22, 4568.

To an oven dried round-bottom flask, the substrate (0.29 mmol, 95% ee) was added. Next, anhydrous DCM (2 mL) and POCl3 (2 mL) were added, and the flask was fitted with a reflux condenser and placed under a nitrogen atmosphere. The resulting solution was allowed to reflux for 4 h. The reaction mixture was then cooled to room temperature and concentrated via rotary evaporation. The resulting residue was dissolved in MeOH/water (3.5 mL, 9:1), which was cooled to 0 ºC. NaBH4 was then added until the pH reached 7. Next, sat. aq. NH4Cl was added dropwise, along with a small chunk of ice. The resulting mixture was diluted with DCM and transferred to a separatory funnel. The organic layer was removed, and the aqueous layer was extracted with DCM. The organic layers were combined, washed with brine, dried over MgSO4, filtered, and concentrated under vacuum. The crude solid was purified via flash column chromatography, and the resulting solid was recrystallized from a minimal amount of boiling ethanol to obtain the cyclized product as a single diastereomer.
Video about Bischler-Napieralski reaction
Images of Bischler-Napieralski reaction
Online database of named reactions
Browse named reactions in alphabetical order or by category in our online database of organic reactions.