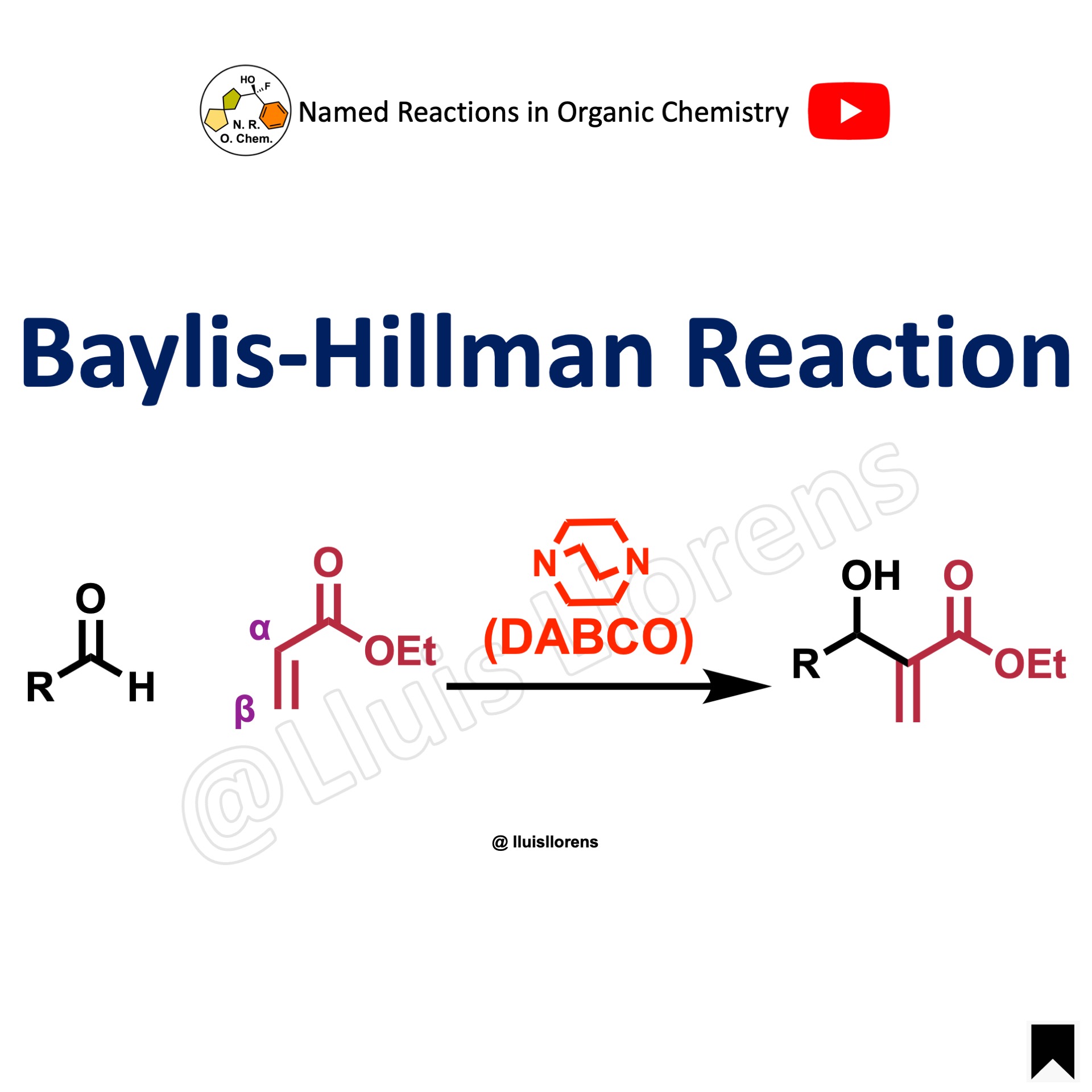
The Baylis-Hillman reaction, sometimes referred to as the Morita-Baylis-Hillman reaction, is a C–C bond-forming reaction between the α-position of an activated alkene (alkyne) and the carbonyl of an aldehyde. It is usually catalyzed by a tertiary amine such as DABCO or phosphine derivatives.
- This transformation provides diverse classes of densely functionalized molecules, which are generally referred to as the Baylis-Hillman adducts.
- Most Baylis-Hillman reactions are catalyzed by organic compounds like tertiary amines and alkyl(aryl) phosphines, and are therefore called “organocatalytic reactions”.
- The Baylis-Hillman reaction is a three-component C–C bond-forming reaction [activated alkenes (alkynes) + electrophiles + catalysts].
- If the substrate contains both the activated alkene and electrophile components in appropriate positions, an intramolecular version of this reaction leading to the synthesis of carbocyclic or heterocyclic compounds may be possible.
- When dealing with a prochiral electrophile, this leads to the formation of a chiral center, presenting both challenges and opportunities for asymmetric development.
- The asymmetric Baylis-Hillman reaction can be mediated efficiently by hydroxylated chiral amines derived from cinchona alkaloids (see example 2).
Reaction mechanism of Baylis-Hillman reaction
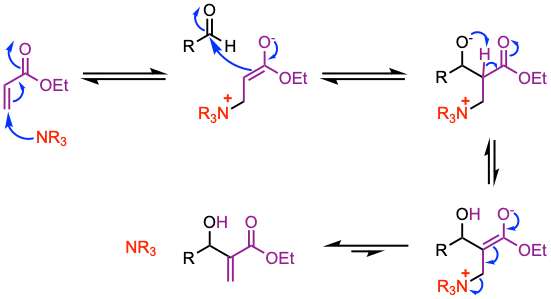
The mechanism involves a Michael addition of the catalyst at the β-position of the activated alkene leading to an enolate that reacts with the carbonyl of an aldehyde to give a zwitterion. The final product is obtained upon release of the catalyst and proton transfer.
1. 1,4-addition of the amine catalyst to the activated alkene.
2. Aldol addition (the enolate adds to the aldehyde).
3. Elimination of the catalyst affords the final product.
For additional details, refer to Chem. Rev. 2010, 110, 5447.
Examples and experimental procedures of Baylis-Hillman reaction
Example 2: Chem. Commun. 2015, 51, 17004.
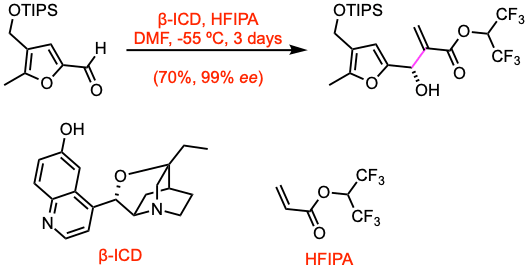
β-Isocupreidine (β-ICD, 0.083 equiv) was dissolved in THF (10 mL), and the solution was evaporated. After repeating this operation three times, the amorphous residue was dried under vacuum at room temperature for 20 min. A solution of the dried β-ICD and the aldehyde (16.9 mmol, 1.0 equiv) in DMF (56 mL) was cooled to -55 oC, and 1,1,1,3,3,3-hexafluoropropan-2- yl acrylate, (HFIPA, 1.3 equiv) was then added. After the mixture was stirred at -55 ºC for 3 days, the reaction mixture was quenched by the addition of 0.1 M HCl. The mixture was extracted with EtOAc, washed with sat. aq. NaHCO3 and brine, dried, and concentrated. The residue was then purified by flash column chromatography to give the desired product with excellent enantiomeric purity.
Example 1: Angew. Chem. Int. Ed. 2021, 60, 12807.
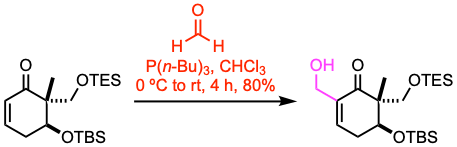
To a stirred solution of the enone (76.6 mmol, 1.0 equiv) and 37% formaldehyde (8.0 equiv) in chloroform (270 mL), tributylphosphine (1.3 equiv) was added dropwise at 0 ºC under nitrogen. The resulting mixture was allowed to warm to room temperature and stirred for 4 h before it was quenched with ice water and extracted with DCM. The combined organic phases were washed with water and brine, dried over anhydrous Na2SO4, filtered, and concentrated under vacuum. The residue was purified by flash column chromatography to afford the β-hydroxy ketone.
Videos about Baylis-Hillman reaction
Images of aldol reaction
Online database of named reactions
Browse named reactions in alphabetical order or by category in our online database of organic reactions.