The Barbier reaction is an organometallic transformation involving the reaction between an alkyl halide, a carbonyl group, and a metal, resulting in the formation of a primary, secondary, or tertiary alcohol. In contrast to the Grignard reaction, the Barbier coupling generates the organometallic species in situ through the reaction between an alkyl halide and a metal. Consequently, the Barbier reaction serves as a valuable alternative to the Grignard reaction, particularly when the generated organometallic species are unstable and cannot be conveniently stored or commercially sold.

- The reaction can be performed using magnesium, zinc, tin, samarium, indium, and some other metals. For SmI2-mediated Barbier reactions in total synthesis, see: Angew. Chem. Int. Ed. 2009, 48, 7140.
- The Barbier reaction as well as the Grignard reaction usually takes place in ethereal solvents, the final alcohol product being obtained by hydrolysis.
Reaction mechanism of Barbier reaction
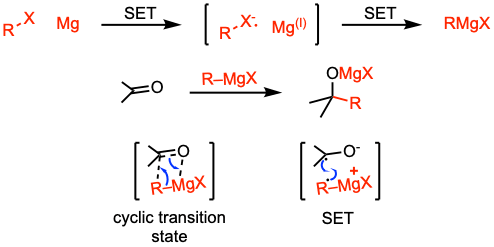
A representation of possible mechanisms for the Barbier reaction: The mechanism of the formation of the organometallic reagent is identical to the formation of a Grignard reagent. The reaction involves a single electron transfer from the metal surface to the alkyl halide. Then, the addition of the Grignard reagent to the carbonyl compound may take place via a concerted process (nucleophilic or polar mechanism) or a radical pathway (radical or single-electron transfer mechanism). Despite more than 100 years of extensive studies, the mechanism of this reaction has remained elusive. For more details on the reaction mechanism, refer to J. Am. Chem. Soc. 2020, 142, 2984 (open access) and J. Phys. Org. Chem. 1989, 2, 205.
Example 1: Angew. Chem. Int. Ed. 2018, 57, 942.

To a solution of the aldehyde (3.83 mmol, 1.0 equiv) in THF/Et2O/H2O (45 mL, 8:1:1), 2,3-dibromopropene (3.0 equiv) and Sn powder (3.0 equiv) were added at room temperature. The resulting mixture was stirred for 2 days until the starting material was fully consumed. The reaction mixture was quenched with sat. aq. NaHCO3 and extracted with EtOAc. The combined organic layers were dried over Na2SO4 and concentrated under reduced pressure to give the crude product as a mixture of diastereomers 1:1, which was used in the next step without further purification.
Videos about Barbier reaction
Images of Barbier reaction
Online database of named reactions
Browse named reactions in alphabetical order or by category in our online database of organic reactions.