The aldol reaction, aldol addition or aldol condensation, involves the addition of the enol or enolate of a carbonyl compound (nucleophile) to an aldehyde or ketone (electrophile). This reaction occurs under either basic or acidic conditions, leading to the formation of β-hydroxy aldehydes or ketones in the case of aldol addition, and α,β-unsaturated aldehydes or ketones in the case of aldol condensation.

Common substrates for the aldol reaction include lithium enolates, enamines, and silyl enol ethers.
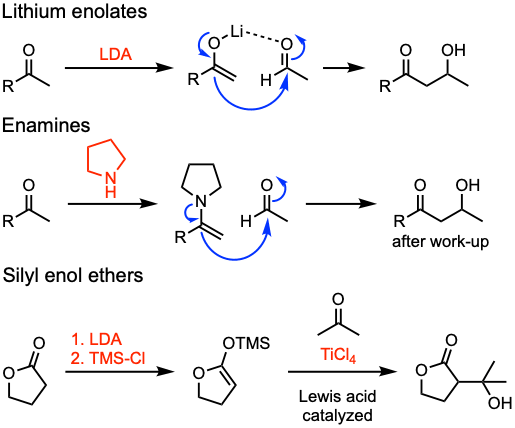
- A diastereoselective aldol addition can be accomplished using chiral auxiliaries (see example 3). Chiral auxiliaries are stereogenic units temporarily incorporated into an organic compound in order to control the stereochemical outcome of the reaction. The chirality present in the auxiliary can bias the stereoselectivity of one or more subsequent reactions. The auxiliary can then be disposed or recovered for future use.
- For an organocatalyst-mediated asymmetric aldol reaction between ethyl glyoxylate and propanal followed by one-pot reduction of the corresponding aldehyde, see example 4.
Reaction mechanism of aldol reaction
In acidic conditions, the mechanism involves the equilibrium formation of an enol that serves as a nucleophile. The carbonyl group of the electrophile is then activated by protonation. In basic conditions, the mechanism involves the formation of an enolate by deprotonation followed by an addition to the carbonyl group. For additional details on the aldol reaction mechanism see, J. Org. Chem. 2016, 81, 5631.

Examples and experimental procedures of aldol reactions
Example 4: Org. Lett. 2023, 25, 4530.
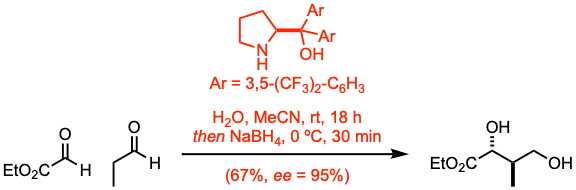
Ethyl glyoxylate polymer (concentrated from 47% toluene solution, 37.5 mmol, 1.0 equiv), was dissolved in acetonitrile (37.5 mL). To this solution were added water (2.06 mL), (S)-2-[bis(3,5-bis-trifluoromethylphenyl)hydroxymethyl]pyrrolidine (10 mol%), and propanal (1.5 equiv). After the reaction mixture was stirred for 18 h at room temperature, a solution of NaBH4 (0.8 equiv) in water (5 mL) was added at 0 ºC. The resulting mixture was stirred for 0.5 h at 0 ºC before the reaction was quenched with sat. aq. NH4Cl. The organic materials were extracted with EtOAc, dried over anhydrous Na2SO4, and concentrated in vacuo after filtration. The crude product was purified by flash column chromatography to give the diol.
Example 3: J. Org. Chem. 2022, 87, 4316.
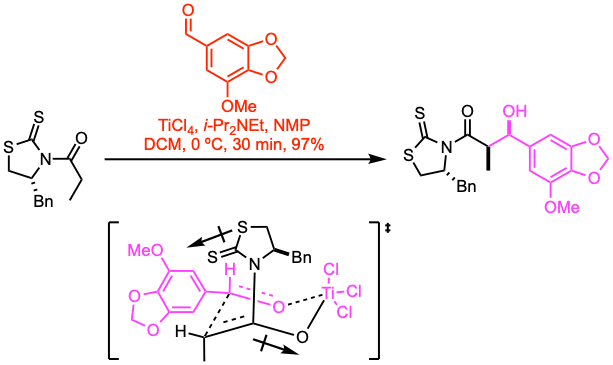
A cooled solution (0 ºC) of the (R)-thiazolidinethione substrate (0.233 mmol, 1.0 equiv) in DCM (2.3 mL, 0.1 M) was treated with titanium(IV) chloride (1.0 M in DCM, 1.1 equiv). After stirring for 15 min at the same temperature, i-Pr2NEt (1.1 equiv) was added dropwise, and the resulting mixture was stirred for 40 min at 0 ºC. 1-methyl-2-pyrrolidinone (NMP, 2.0 equiv) was added, and the mixture was stirred for an additional 10 min. 5- methoxypiperonal (3.0 equiv) in DCM (2 mL) was added to the enolate, and the resulting mixture was stirred for 30 min before it was quenched by the addition of sat. aq. NH4Cl, and diluted with DCM. The layers were separated, and the aqueous layer was extracted with DCM. The combined organic layers were washed with brine, dried over anhydrous Na2SO4, and concentrated under vacuum. The residue was purified by flash column chromatography to provide the syn-aldol adduct.
Example 2: Angew. Chem. Int. Ed. 2020, 59, 23609.
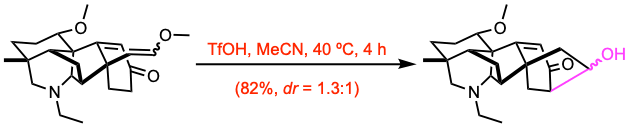
The enone (0.156 mmol, 1.0 equiv) was dissolved in MeCN (58 mL) followed by the addition of TfOH (20 equiv). The reaction mixture was heated to 40 ºC and stirred for 4 h. Upon completion, the mixture was cooled to room temperature before it was quenched with sat. aq. NaHCO3. The mixture was extracted with EtOAc, washed with brine, dried over Na2SO4, and concentrated. Subjection of the residue to chromatography purification on silica gel afforded the alcohol.
Example 1: Angew. Chem. Int. Ed. 2020, 59, 23609.

To a solution of the racemic diketone (1.09 mmol, 1.0 equiv) in THF (85 mL), LDA (2 M in THF/hexane, 5.0 equiv) was slowly added at -78 ºC. After stirring for 20 min at the same temperature, the reaction mixture was quenched with sat. aq. NH4Cl, warmed to room temperature, and extracted with EtOAc. The combined organic layers were washed with brine, dried over anhydrous Na2SO4, filtered, and concentrated under vacuum. The crude residue was then purified by flash column chromatography to afford the racemic β-hydroxy ketone.
Videos about aldol reaction
Images of aldol reaction
Online database of named reactions
Browse named reactions in alphabetical order or by category in our online database of organic reactions.