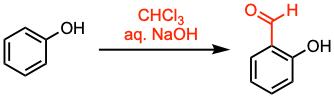
The Reimer-Tiemann reaction facilitates the formylation of phenols using chloroform and a strong base, typically an aqueous hydroxide solution.
The reaction proceeds via electrophilic aromatic substitution with the formed dichlorocarbene. While the regioselectivity is not high, ortho-formyl products are the predominant outcome. Under the original Reimer-Tiemann conditions, pyrroles undergo ring-expansion to produce 3-chloropyridines, a process known as the Ciamician-Dennstedt rearrangement.
- The reaction is an electrophilic aromatic substitution that occurs under basic conditions in a protic solvent.
- The substrate is dissolved in 10–40% alkali hydroxide, excess chloroform is added, and the biphasic solution is vigorously stirred at elevated temperatures.
- When the reaction is conducted in the presence of cyclodextrins, or if the ortho-position is already substituted, the p-formyl product is formed predominantly.
- For further details on the Reimer-Tiemann reaction, see: (i) Chem. Rev. 1960, 60, 169. (ii) Wynberg, H. and Meijer, E.W. (2005). The Reimer–Tiemann Reaction. In Organic Reactions, (Ed.). (iii) Org. Biomol. Chem. 2011, 9, 5109. (iv) Synth. Commun. 2011, 41, 3678.
Reaction mechanism of Reimer-Tiemann reaction
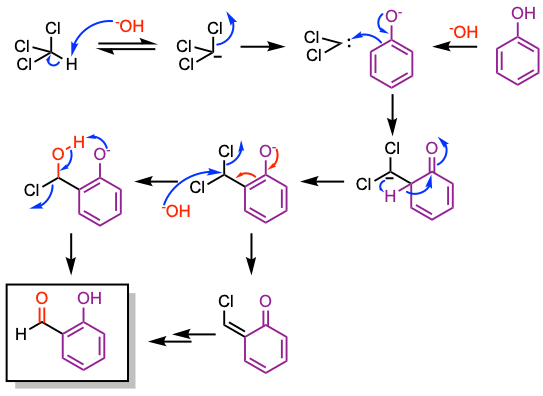
1. Chloroform is deprotonated by the base to generate the chloroform carbanion.
2. Alpha-elimination gives dichlorocarbene.
3. Nucleophilic attack by the phenoxide ion leads to a series of intermediates that provide the desired product upon basic hydrolysis.
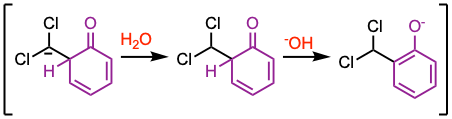
The 1,2-proton shift appears unlikely according to Hückel and orbital-symmetry calculations. Tritium isotope experiments showed complete transfer of the isotope from tritium oxide to the formyl group. Proton exchange between water and the aldehydic proton is absent under the reaction conditions. Thus, these experiments favor a mechanism involving the formation of a neutral intermediate. For additional details, see J. Org. Chem. 1971, 36, 202.
Examples and experimental procedures of Reimer-Tiemann reactions
Example 1: Org. Lett. 2007, 9, 1367.
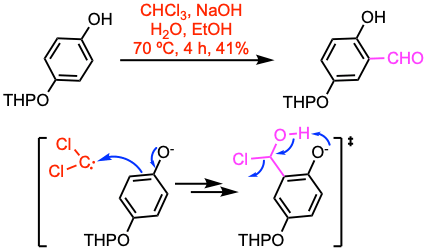
A solution of the phenol (30.9 mmol, 1.0 equiv) and sodium hydroxide (8.0 equiv) in ethanol/H2O (100 mL, 2:1) was heated to 70 ºC, chloroform (2.0 equiv) was added over 1 h. The resulting mixture was stirred for 3 h, cooled to room temperature, and evaporated to remove ethanol. The remaining aqueous solution was adjusted to pH 4~5 and extracted with EtOAc. Standard workup and purification procedures gave the desired aldehyde.
Video about Reimer-Tiemann reaction
Images of Reimer-Tiemann reaction
Online database of named reactions
Browse named reactions in alphabetical order or by category in our online database of organic reactions.