The Wolff-Kishner reduction allows the deoxygenation of aldehydes and ketones to hydrocarbons via the corresponding hydrazones under basic conditions and heat. The reaction is commonly employed to remove a carbonyl group after it has served its synthetic purpose in preceding steps. The reaction is usually carried out in high boiling solvents (e.g., DGME).

- Sterically hindered carbonyl compounds are deoxygenated more slowly.
- Since the reaction requires highly basic conditions, it is unsuitable for base-sensitive substrates.
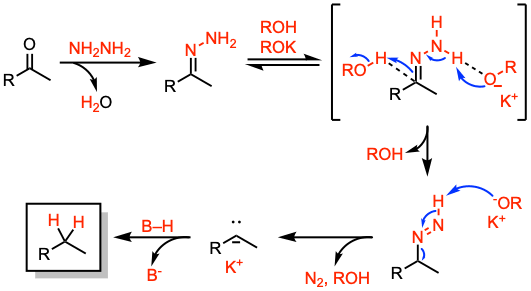
Reaction mechanism of Wolff-Kishner reduction
A suggested mechanism involves the formation of a hydrazone anion by deprotonation of the terminal nitrogen by the base. The rate-determining step is the proton capture at the carbon terminal. For more details on the reaction mechanism, see: Angew. Chem. Int. Ed. Engl. 1968, 7, 120.
Examples and experimental procedures of Wolff-Kishner reduction
Example 3: Org. Lett. 2023, 25, 4530.

To a solution of KOH (10.6 equiv) and NH2NH2·H2O (5.0 equiv) in ethylene glycol (26 mL), isovanillin (13.1 mmol, 1.0 equiv) was added at room temperature. After the reaction mixture was stirred at 130 ºC for 1 h, the temperature was increased to 190 ºC, and the reaction mixture was stirred at the same temperature for an additional 5 h. After the reaction mixture was cooled to room temperature, the resulting mixture was poured into a mixture of ice and aqueous HCl. The organic materials were extracted with Et2O, dried over anhydrous Na2SO4, and concentrated in vacuo after filtration to give the deoxygenated product, which may be used without further purification.
Example 2: J. Am. Chem. Soc. 2023, 145, 21170.

Example 1: Angew. Chem. Int. Ed. 2021, 60, 14967.
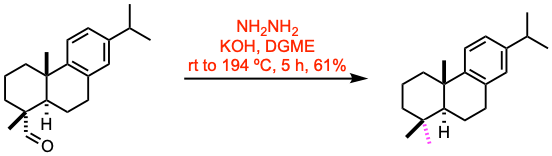
To a stirred solution of the aldehyde (1.1 mmol, 1.0 equiv) and hydrazine monohydrate (20.0 equiv) in diethylene glycol monomethyl ether (DGME, 22 mL), KOH (6.0 equiv) was added at room temperature. The resulting mixture was heated at 110 ºC for 1 h, at 194 ºC for 4 h, and was then cooled to room temperature. The mixture was quenched by the addition of 1.0 M aq. HCl, and extracted with Et2O. The combined organic layers were dried over Na2SO4, filtered, and evaporated to dryness. The resulting residue was purified by flash column chromatography to afford the deoxygenated product.
Videos about Wolff-Kishner reduction
Images of Wolff-Kishner reduction
Online database of named reactions
Browse named reactions in alphabetical order or by category in our online database of organic reactions.