

by Lluis Llorens | Sep 29, 2022 | Named Reactions
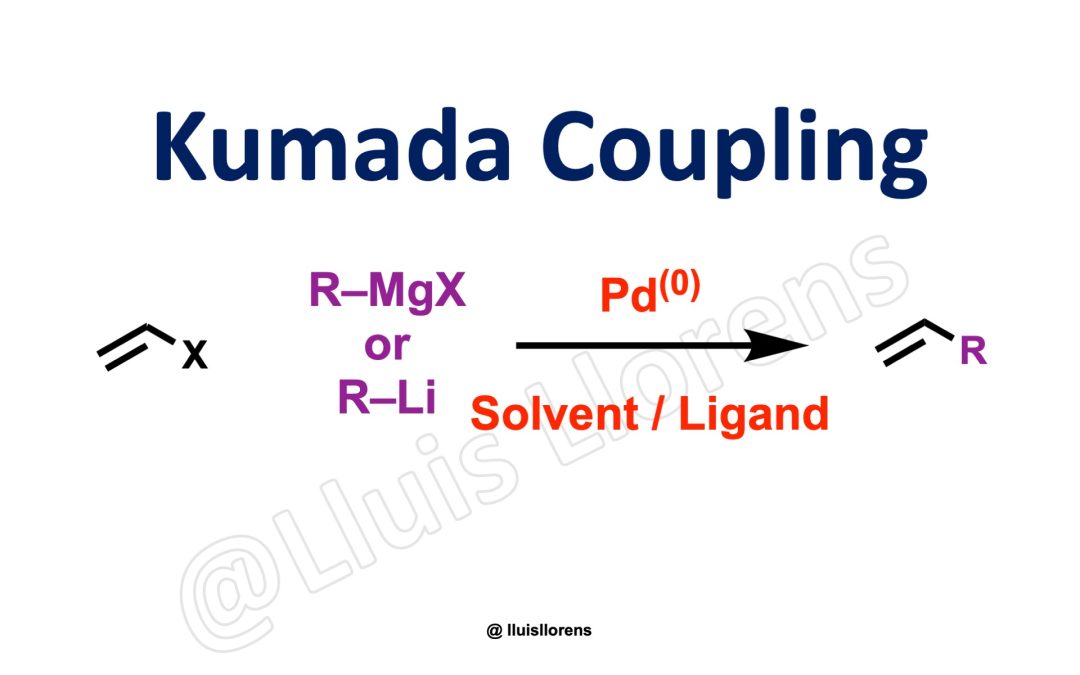
FollowFollowFollow Kumada Coupling The Kumada cross-coupling is the transition metal-catalyzed carbon-carbon bond-forming reaction of alkenyl or aryl halides and organomagnesium or organolithium reagents. General features: 1. The coupling is stereoselective, and the...Search
Topics
Resources for chemists
 Ebook Bundle: The Chemists’ Cookbook ebook + Named Reactions Handbook & Quiz ebook
Ebook Bundle: The Chemists’ Cookbook ebook + Named Reactions Handbook & Quiz ebook
 Named Reactions Handbook & Quiz - Lluís Llorens Palomo
Named Reactions Handbook & Quiz - Lluís Llorens Palomo
 The Chemists’ Cookbook ebook - Lluís Llorens Palomo
The Chemists’ Cookbook ebook - Lluís Llorens Palomo

We use cookies to keep your experience smooth, secure, and personalized. You’re always in control—you can manage your preferences anytime. For the best experience, we recommend enabling all. Privacy Policy
| Name | Domain | Purpose | Expiry | Type |
|---|---|---|---|---|
| wpl_user_preference | nrochemistry.com | WP GDPR Cookie Consent Preferences. | 1 year | HTTP |
| YSC | youtube.com | YouTube session cookie. | 55 years | HTTP |
| Name | Domain | Purpose | Expiry | Type |
|---|---|---|---|---|
| VISITOR_INFO1_LIVE | youtube.com | YouTube cookie. | 6 months | HTTP |
| Name | Domain | Purpose | Expiry | Type |
|---|---|---|---|---|
| _ga | nrochemistry.com | Google Universal Analytics long-time unique user tracking identifier. | 2 years | HTTP |
| sbjs_migrations | nrochemistry.com | Sourcebuster tracking cookie | 55 years | HTTP |
| sbjs_current_add | nrochemistry.com | Sourcebuster tracking cookie | 55 years | HTTP |
| sbjs_first_add | nrochemistry.com | Sourcebuster tracking cookie | 55 years | HTTP |
| sbjs_current | nrochemistry.com | Sourcebuster tracking cookie | 55 years | HTTP |
| sbjs_first | nrochemistry.com | Sourcebuster tracking cookie | 55 years | HTTP |
| sbjs_udata | nrochemistry.com | Sourcebuster tracking cookie | 55 years | HTTP |
| sbjs_session | nrochemistry.com | SourceBuster Tracking session | Session | HTTP |
| Name | Domain | Purpose | Expiry | Type |
|---|---|---|---|---|
| NID | google.com | Google unique id for preferences. | 6 months | HTTP |
| Name | Domain | Purpose | Expiry | Type |
|---|---|---|---|---|
| VISITOR_PRIVACY_METADATA | youtube.com | --- | 6 months | --- |
| woocommerce_geo_hash | nrochemistry.com | --- | Session | --- |
| _ga_Q47NGCVMY8 | nrochemistry.com | --- | 2 years | --- |