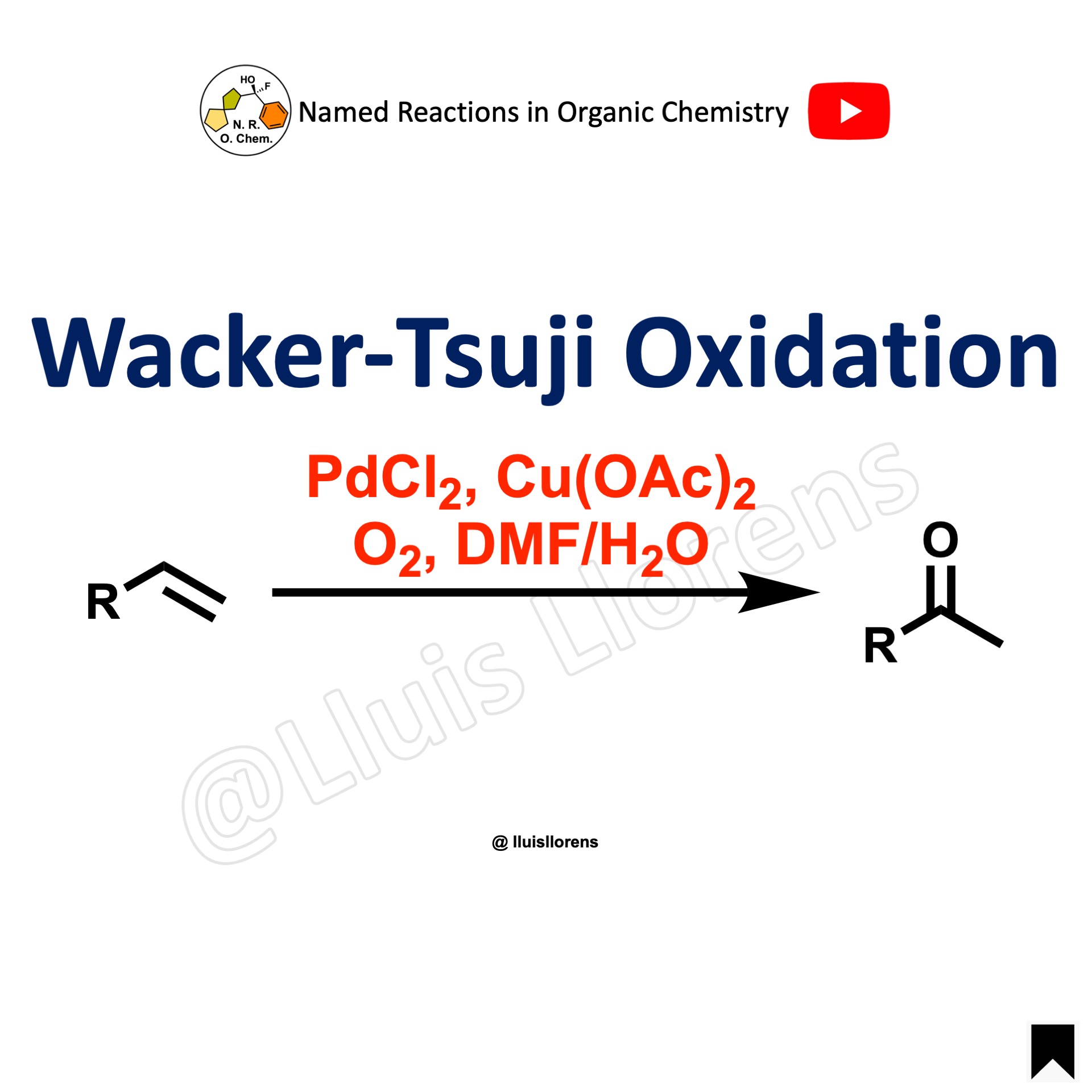
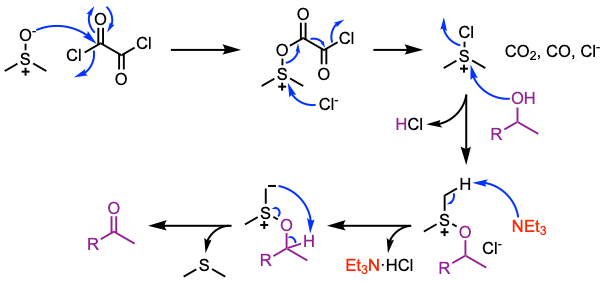
The Swern oxidation allows the preparation of aldehydes and ketones from primary and secondary alcohols. DMSO reacts vigorously with oxalyl chloride when no solvent is present; it can lead to an explosion. The most common solvent for this transformation is DCM. The oxidation is usually conducted at -78 ºC to stabilize the intermediates of the reaction.
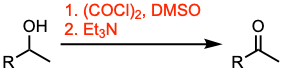
- Typical procedure: Reaction of DMSO with oxalyl chloride at low temperature followed by the slow addition of the alcohol. Then, the tertiary amine is added to facilitate the decomposition of the alkoxysulfonium salt leading to the desired product.
- Similar oxidation reactions include the Corey-Kim oxidation and the Parikh-Doering oxidation.
Reaction mechanism of Swern oxidation
(i) Activation of DMSO with oxalyl chloride. (ii) Formation of the chlorosulfonium salt. (iii) Activation of the alcohol and formation of an alkoxysulfonium salt. (iv) Deprotonation and formation of an alkoxysulfonium ylide. For mechanistic insights into the Swern oxidation and further applications of oxalyl chloride in organic synthesis, see: ChemistrySelect 2019, 4, 6309.
Examples and experimental procedures of Swern oxidation
Example 2: J. Am. Chem. Soc. 2024, 146, 8456.

To a cold (-78 ºC), stirred solution of (COCl)2 (1.2 equiv) in DCM (40 mL) was added DMSO (2.4 equiv) dropwise over 5 min. (caution! CO/CO2 (g) liberated), and the reaction mixture was stirred for 10 min at -78 ºC. After this time, a solution of the alcohol (8.39 mmol, 1.0 equiv) in DCM (10 mL + 2×5 mL rinse) was slowly added via syringe over 10 min, and the reaction mixture was maintained at -78 ºC for a further 20 min. After this time, Et3N (5.0 equiv) was added via syringe, and the reaction mixture was maintained at -78 ºC for a further 10 min. The reaction mixture was then warmed to room temperature and stirred for a further 1 h 30 min. After this time, water was added and aqueous HCl (1 M) was added until the aqueous layer had a pH of ~4 as determined by pH paper. The resulting biphasic mixture was poured into a separatory funnel and the organic layer was separated and washed with H2O and brine. The combined aqueous layers were extracted with Et2O. The combined Et2O and DCM extracts were dried over MgSO4, filtered, and the solvent was removed in vacuo. This process afforded the crude aldehyde, which was immediately used in the subsequent step without further purification.
Example 1: Angew. Chem. Int. Ed. 2020, 59, 13990.
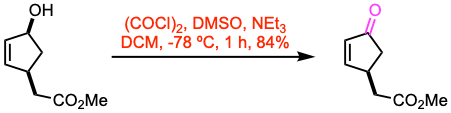
To a solution of oxalyl chloride (1.5 equiv) in DCM (36 mL) was added a solution of dimethylsulfoxide (2.7 equiv) in DCM (15 mL) at -78 ºC over 5 min. After 5 min, a solution of the secondary alcohol (24.0 mmol, 1.0 equiv) in DCM (24 mL) was added dropwise over 5 min. After 30 min at -78 ºC, triethylamine (7.0 equiv) was added dropwise over 10 min. The mixture was allowed to warm-up to room temperature, and water was added. The product was extracted with DCM. The combined organic layers were washed with brine, dried over Na2SO4, and concentrated. Purification by flash column chromatography afforded the ketone.
Video about Swern oxidation
Images of Swern oxidation
Online database of named reactions
Browse named reactions in alphabetical order or by category in our online database of organic reactions.