The Suzuki coupling, or Suzuki–Miyaura reaction, is a palladium-catalyzed cross-coupling between organoboronic acids or esters and organic halides or triflates under basic conditions to form a new C–C sigma bond.
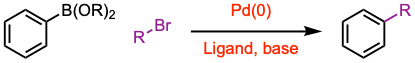
- The Suzuki coupling reaction is performed between an aryl halide (R-X) as an electrophile and an organoborane (R-B) as a nucleophile with a metal catalyst, suitable base, aqueous and organic solvents, and temperature (usually 60–100 ºC), generally to make biaryl products.
- Several solvents classified as polar and non-polar can be used in the Suzuki coupling reaction, such as N,N-dimethylformamide (DMF), tetrahydrofuran (THF, see example 1), dimethyl sulfoxide (DMSO), 1,4-dioxane (see example 3), carbon tetrachloride (CCl4), water (H2O), methanol (MeOH), ethyl alcohol (EtOH), and toluene (PhMe, see example 4).
- For an sp2-sp3 Suzuki cross-coupling reaction (see example 2).
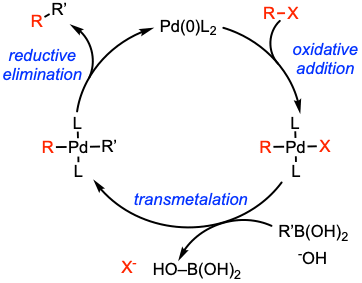
Reaction mechanism of Suzuki coupling
A general catalytic cycle. The Suzuki–Miyaura cross-coupling reactions follow a well-defined catalytic cycle based on three steps: (i) oxidative addition of the palladium(0) complex to the organic halide to give R–Pd(II)–X; (ii) transmetalation between R–Pd(II)–X and the boronate (R’– BY2) with the assistance of a base; and finally, (iii) reductive elimination to form a new C–C bond with simultaneous regeneration of the catalyst. For mechanistic aspects, see: Chem. Eur. J. 2021, 27, 13481. Open access.
Examples and experimental procedures of Suzuki couplings
Example 4: J. Am. Chem. Soc. 2022, 144, 12970.

To a flame-dried test-tube, the vinyl iodide (0.265 mmol, 1.0 equiv), Pd(OAc)2 (0.1 equiv), SPhos (0.2 equiv), K3PO4 (4.0 equiv), and MeB(OH)2 (5.0 equiv) were added. The tube was sealed with a septum and purged five times using alternating vacuum and argon refills. Then, degassed toluene (3.5 mL), water (10.0 equiv), and THF (0.48 mL) were added. The mixture was stirred at room temperature for 5 min before it was heated to 80 ºC in an oil bath for 1 h. UPC2-MS showed complete consumption of the starting material. The tube was removed from the oil bath, and the reaction mixture was cooled to room temperature. The mixture was filtered through Celite and thoroughly washed with EtOAc. The filtrate was concentrated, and the residue was purified by flash column chromatography to afford the coupled product.
Example 3: J. Am. Chem. Soc. 2021, 143, 21270.
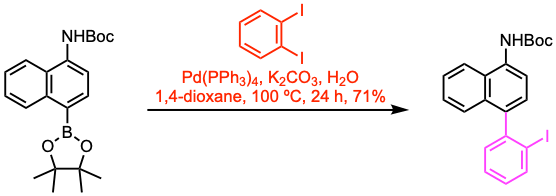
A round-bottom flask with a stir bar was fitted with a rubber septum and flame-dried under high vacuum. The flask was purged with argon and charged with the substrate (7.3 mmol, 1.0 equiv), Pd(PPh3)4 (0.055 equiv), 1,2-diiodobenzene (1.5 equiv), K2CO3 (2.0 equiv), and a deoxygenated solution of 1,4-dioxane/water (20/5 mL). The resulting solution was stirred at 100 ºC for 24 h. After completion, the reaction mixture was cooled to room temperature. Water was added, and the mixture was extracted with EtOAc. The organic phase was dried over anhydrous MgSO4 and concentrated under reduced pressure. The residue was purified by flash column chromatography to afford the coupled product.
Example 2: J. Am. Chem. Soc. 2021, 143, 2710.

Example 1: Angew. Chem. Int. Ed. 2021, 60, 12392.
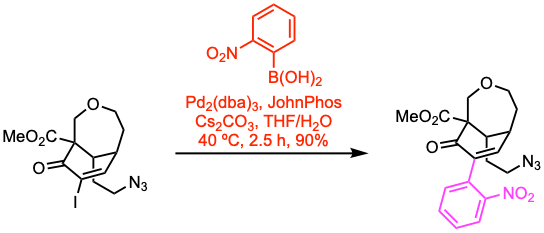
A round-bottom flask was charged with the organic halide (0.22 mmol, 1.0 equiv), the boronic acid (1.1 equiv), Pd2(dba)3 (0.05 equiv), 2-(ditert– butylphosphino)-biphenyl (JohnPhos, 0.2 equiv), and cesium carbonate (3.0 equiv). THF (2.5 mL) and water (0.5 mL) were sequentially added to the flask, and the resulting mixture was heated to 40 ºC under an argon atmosphere for 2.5 h. After completion, the reaction mixture was allowed to cool to room temperature, quenched with sat. aq. NH4Cl, and extracted with EtOAc. The combined organic extracts were washed with brine, dried over Na2SO4, filtered, and concentrated under vacuum. The crude product was purified by flash column chromatography to afford the coupled product.
Videos about Suzuki coupling
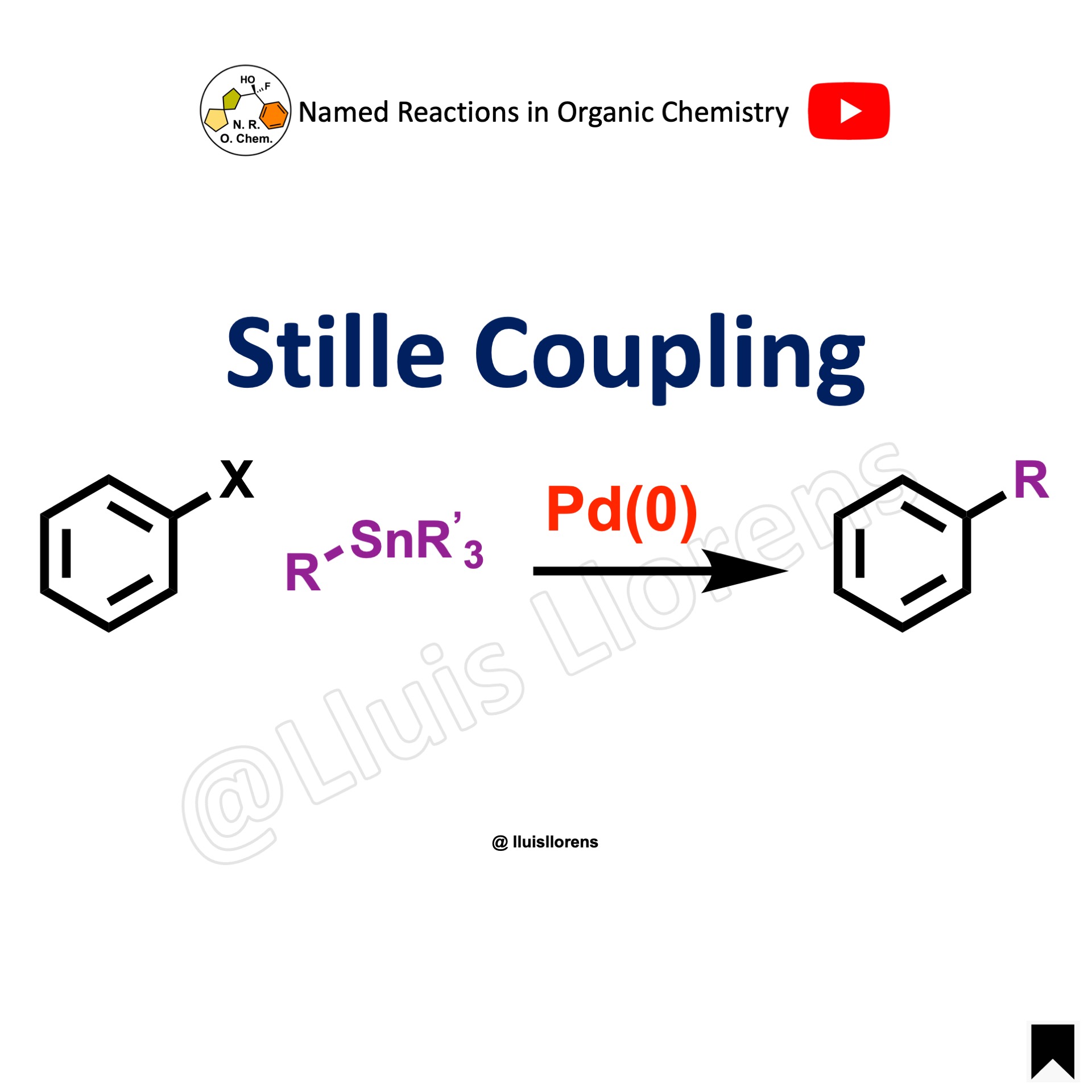
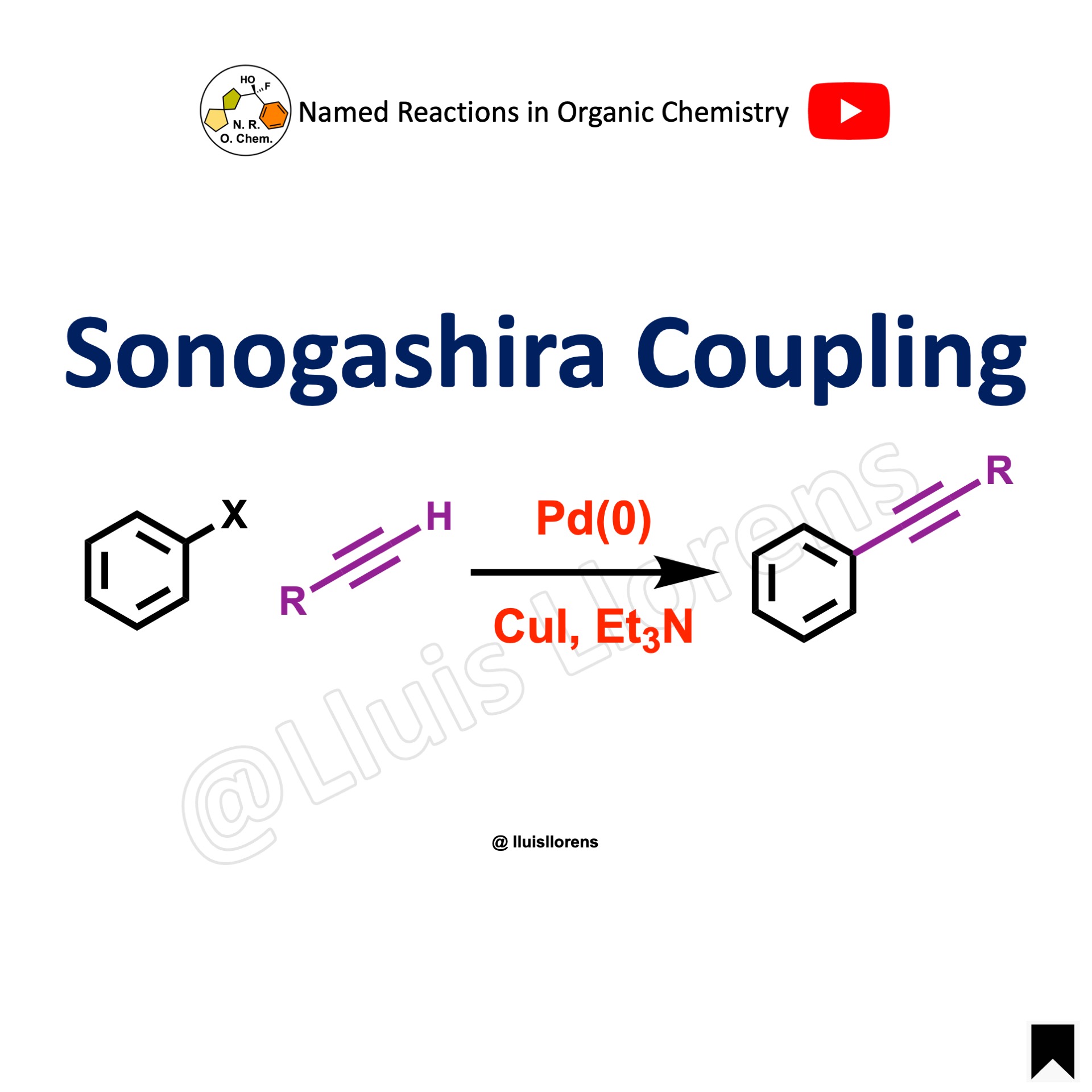
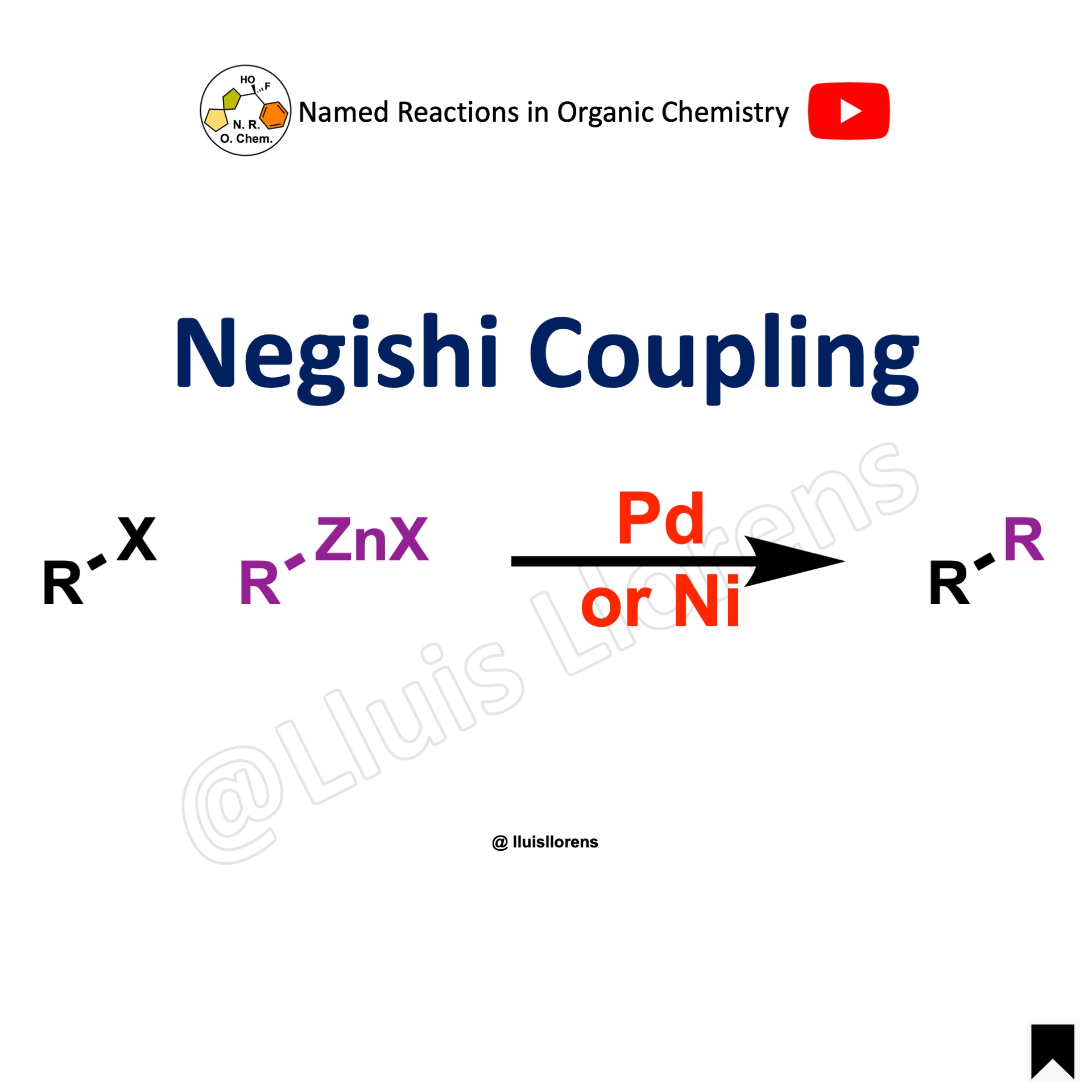
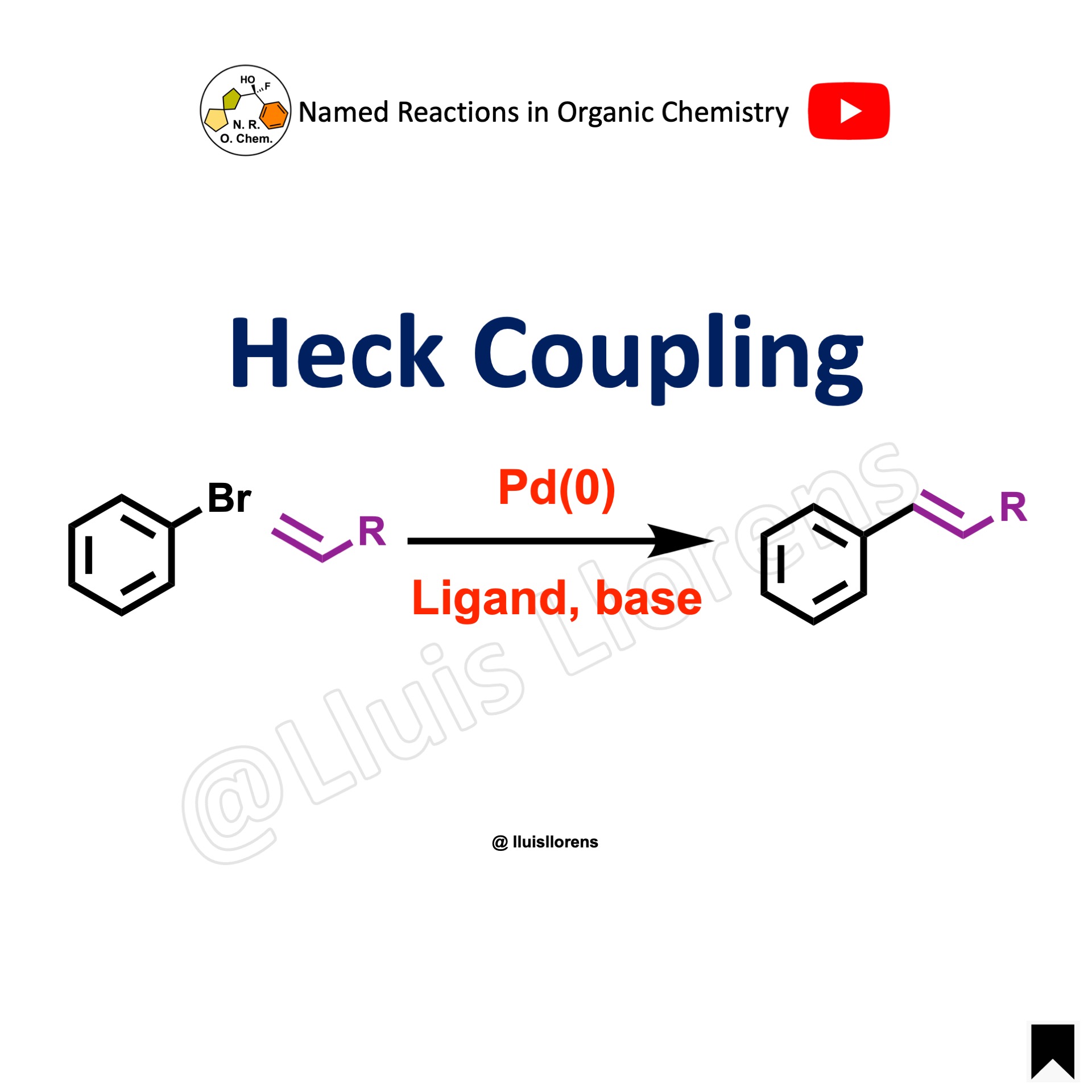
Images of Suzuki coupling
Online database of named reactions
Browse named reactions in alphabetical order or by category in our online database of organic reactions.