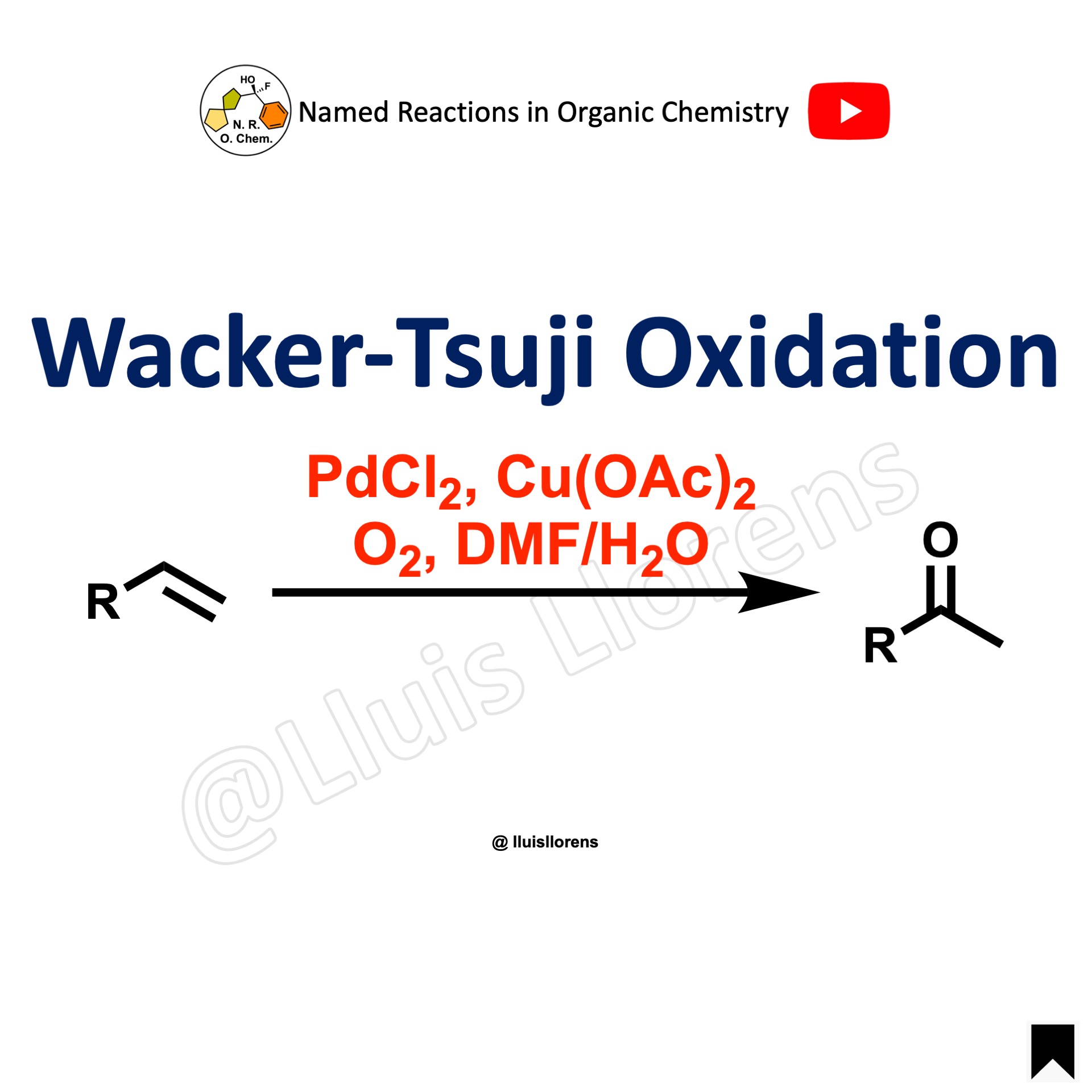
In the Pinnick oxidation, aldehydes are oxidized into their corresponding carboxylic acids using sodium chlorite, mild acidic conditions, and a scavenger. The reaction is also known as Pinnick-Kraus, Lindgren-Kraus, or Pinnick-Lindgren-Kraus oxidation.
Despite the reaction is commonly referred to as the Pinnick oxidation, Tetrahedron 1981, 37, 2091, the reaction conditions were first developed by Kraus. See the seminal publications in J. Org. Chem. 1980, 45, 4825 and J. Org. Chem. 1980, 45, 1175. The preparation of carboxylic acids from aldehydes by oxidation with chlorites was also developed by Lindgren, see: Acta Chem. Scand. 1973, 27, 888.
- The typical solvent is a combination of tert-butanol with another solvent (e.g., THF, water)
- The use of an excess of a scavenger (e.g., 2-methyl-2-butene) is needed. HClO is formed as a by-product of the oxidation process, and it can cause side reactions such as the consumption of the oxidizing agent NaClO2 to form chlorine oxide (ClO2). To avoid this sabotaging side reaction, scavengers provide a double bond that consumes the generated by-product HClO.
- The reaction tolerates a wide range of functional groups.
- The Pinnick oxidation is a convenient method among oxidation reactions for converting aldehydes into carboxylic acids.
Reaction mechanism of Pinnick oxidation

1. Formation of chlorous acid.
2. The chlorous acid adds to the aldehyde.
3. The resulting intermediate undergoes pericyclic reaction.
4. Formation of the carboxylic acid and hypochlorous acid.
5. The scavenger consumes the hypochlorous acid to avoid side reactions with the starting material.
For additional mechanistic investigations on the Pinnick oxidation, see: R. Soc. Open Sci. 2020, 7, 191568. Open access.
Examples and experimental procedures of Pinnick oxidation
Example 3: J. Am. Chem. Soc. 2023, 145, 5001.

To a stirred solution of the aldehyde (2.25 mmol, 1.0 equiv) in tetrahydrofuran/water/tert-butanol (45 mL, 4:4:1) were added 2-methyl-2-butene (15.0 equiv) at 0 ºC, followed by the sequential addition of sodium dihydrogen phosphate monohydrate (6.0 equiv) and sodium chlorite (80% purity, 2.8 equiv). The resulting reaction mixture was stirred at 0 ºC for 4 h. TLC showed that the starting material was consumed completely. The reaction mixture was diluted with brine and extracted with DCM. The combined organic layers were dried over anhydrous sodium sulfate, filtered, and concentrated at 0 ºC under vacuum. The residue was redissolved in a small amount of DCM and filtered through a pad of anhydrous Na2SO4. The filtrate was concentrated at 0 ºC under vacuum to give the carboxylic acid which was directly used in the next step without further purification.
Example 2: Angew. Chem. Int. Ed. 2022, 61, e202200576.

To a solution of the aldehyde (22.8 mmol, 1.0 equiv) in DCM (150 mL) was added DMP (1.20 equiv). The solution was stirred at room temperature for 2 hours before it was diluted with Et2O (300 mL). The mixture was filtered through a short pad of silica gel, and the filter residue was washed with Et2O/EtOAc (1 L, 2:1). The filtrate was concentrated in vacuo giving the residue, which was used directly in the next step without further purification. The residue was re-dissolved in t-BuOH/H2O (300 mL, 3:2) and cooled to 0 ºC. To the solution, 2-methyl-2-butene (7.0 equiv), NaH2PO4·2H2O (2.0 equiv), and NaClO2 (2.0 equiv) were added. The reaction mixture was stirred for 1 hour at 0 ºC before it was quenched with sat. aq. Na2SO3. The layers were separated, and the aqueous phase was extracted with EtOAc. The organic extracts were combined, dried with Na2SO4, and concentrated in vacuo. Purification of the residue by FCC gave the carboxylic acid.
Example 1: Angew. Chem. Int. Ed. 2020, 59, 6253.

To a stirred solution of the aldehyde (0.636 mmol, 1.0 equiv) in t-BuOH (3.0 mL) and H2O (3.0 mL), 2-methyl-2-butene (20.0 equiv), NaH2PO4 (10.0 equiv), and NaClO2 (10.0 equiv) were added at room temperature. After stirring for 14 h, the mixture was quenched with aq. NaHSO3 at room temperature and extracted with EtOAc. The combined organic layer was dried over Na2SO4. After filtration, removal of the solvent under reduced pressure gave a crude material, which was purified by flash column chromatography to afford the corresponding carboxylic acid with retention of configuration.
Videos about Pinnick oxidation
Images of Pinnick oxidation
Online database of named reactions
Browse named reactions in alphabetical order or by category in our online database of organic reactions.