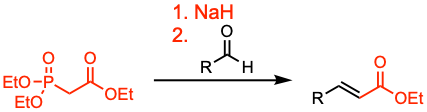
The Horner-Wadsworth-Emmons (HWE) reaction allows the stereoselective olefination of aldehydes and ketones using stabilized phosphonate carbanions. The reaction favors the formation of (E)-alkenes.
The Still-Gennari conditions (see example 2 and example 7) allow the preparation of (Z)-olefins with excellent stereoselectivity by the reaction between aldehydes and phosphonates bearing electron-withdrawing groups (e.g., trifluoromethyl). The use of electron-withdrawing phosphonates accelerates the elimination of the oxaphosphetane intermediates, boosting the production of (Z)-alkenes.
Phosphonate carbanions are more nucleophilic than the corresponding phosphorus ylides, allowing them to react readily with a wide range of aldehydes and ketones under milder conditions. Hindered ketones that are typically unreactive in Wittig reactions can react readily in HWE olefinations. See Tetrahedron Lett. 2018, 59, 568, Synthesis 2021, 53, 2713, and Org. Biomol. Chem. 2023, 21, 1095 for more details.
- The preparation of starting alkyl phosphonates is possible using the Arbuzov reaction.
- For a cross metathesis approach to access allylic phosphonates, see example 5.
- In addition to its applicability to macrocyclization, the HWE reaction is useful for the formation of small rings (five- to seven-membered rings, see example 1 and example 4).
- The HWE reaction is one of the most reliable methods for carbon chain elongation (see example 3) and for the synthesis of α,β-unsaturated carbonyl groups.
- The Masamune-Roush olefination conditions make use of lithium chloride and an amine for base-sensitive compounds in a Horner-Wadsworth-Emmons reaction (see example 6).
Reaction mechanism of Horner-Wadsworth-Emmons reaction
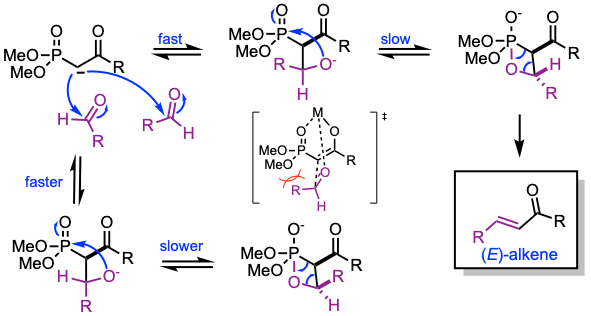
1. Deprotonation of the phosphonate.
2. Nucleophilic addition of the carbanion onto the aldehyde.
3. Formation of an oxaphosphetane intermediate.
4. Final elimination delivers predominantly the (E)-olefin, or the (Z)-olefin when the Still-Gennari conditions are employed instead.
The HWE reaction occurs with the addition of the phosphonate enolate to the aldehyde, followed by oxaphosphetane formation (pseudorotation, P−C bond cleavage and then O−C bond cleavage).
The oxaphosphetane formation is the rate-determining step, in which the transition state leading to trans-olefin is lower in energy than that leading to cis-olefin.
For more details on the Horner-Wadsworth-Emmons reaction mechanism, see J. Org. Chem. 1999, 64, 6815.
Examples and experimental procedures of Horner-Wadsworth-Emmons reactions
Example 7: J. Am. Chem. Soc. 2024, 146, 8456.
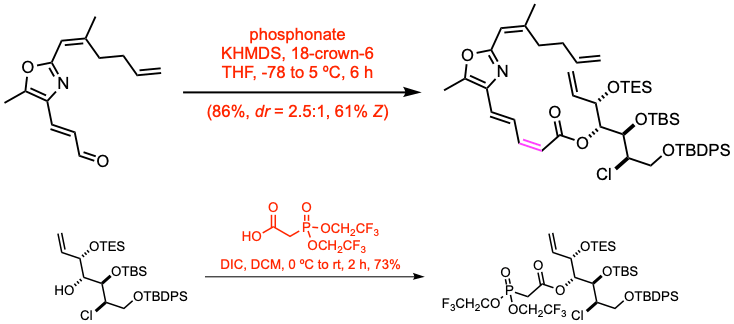
18-crown-6 solution was dried for 2 days over 20% (m/v) 3 Å molecular sieves prior to use in this reaction. To a cold (-78 ºC), stirred solution of the phosphonate (3.83 mmol,1.0 equiv) in THF (9.5 mL) was added in sequence 18-crown-6 (1.0 M in THF, 2.0 equiv) and KHMDS (1 M in THF, 1.02 equiv), and the resulting slurry was vigorously stirred at -78 ºC for 1 h 15 min. After this time, the reaction mixture was warmed to -46 ºC and the aldehyde (1.0 equiv) was added as a solution in THF (0.8 mL + 2×0.8 mL rinses) and the reaction mixture was maintained at -46 ºC for a further 3 h. After this time, the cold bath was allowed to gradually warm to 5 ºC over the course of an additional 2 h. After this time, the reaction mixture was quenched by the addition of sat. aq. NH4Cl, and the resulting mixture was poured into a separatory funnel and extracted with DCM. The combined organic layers were dried over Na2SO4, filtered, and the solvent was removed in vacuo. The crude product was then purified via flash column chromatography.
Example 6: J. Am. Chem. Soc. 2024, 146, 8456.
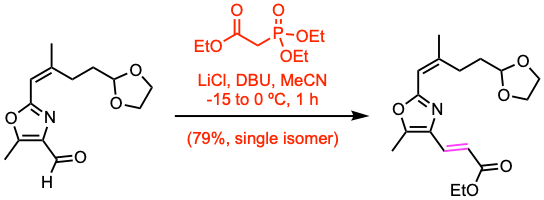
LiCl was flame dried in vacuo immediately prior to use in this procedure. To a cold (-15 ºC), vigorously stirred suspension of the aldehyde (13.9 mmol, 1.0 equiv), LiCl (1.6 equiv), and triethylphosphonoacetate (1.5 equiv) in MeCN (70 mL) was added DBU (1.5 equiv) via syringe, and the reaction mixture was allowed to slowly warm over the course of 1 h to 0 ºC. After this time, the reaction mixture was allowed to warm to room temperature and stirred for an additional 10 min. The reaction mixture was quenched by the addition of a sat. aq. NH4Cl, and H2O was added until all solids dissolved. The resulting mixture was poured onto DCM in a separatory funnel, shaken, and the organic layer was separated. The aqueous layer was extracted with DCM, and the combined organic layers were dried over Na2SO4, filtered, and the solvent was removed in vacuo. The crude product was purified via flash column chromatography.
Example 5: J. Am. Chem. Soc. 2025, 147, 3010.
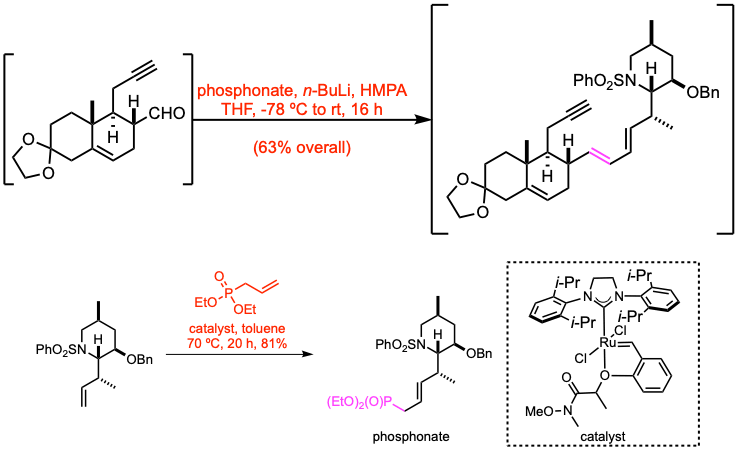
In a Schlenk flask with stir bar, allyl phosphonate (0.213 mmol, 1.0 equiv) was dissolved in THF (1.1 mL) and cooled to -78 ºC. n-Butyl lithium (2.5 M in hexanes, 1.1 equiv) was added dropwise, and the reaction mixture was stirred for 15 minutes. After 15 minutes, the crude aldehyde dissolved in THF (0.55 mL, 0.40 M, 1.0 equiv) containing hexamethylphosphoramide (2.4 equiv) was added dropwise to the allyl phosphonate solution. The reaction mixture was kept at -78 ºC for 4 h then slowly warmed to room temperature over approximately 4 h, after which the reaction was stirred for an additional 8 h.
Example 4: Angew. Chem. Int. Ed. 2022, 61, e202210297.

Lithium bromide (10.0 equiv) and triethylamine (4.0 equiv) were added to a solution of the aldehyde (5.67 mmol, 1.0 equiv) in dry THF (100 mL), under an argon atmosphere, and the reaction mixture was stirred at room temperature for 3h. The reaction mixture was partitioned between ethyl acetate and brine, the organic extract was dried over anhydrous MgSO4, filtered, and concentrated in vacuo. The residue was purified by dry-flash chromatography to give the tricyclic product.
Example 3: J. Am. Chem. Soc. 2022, 144, 5253.
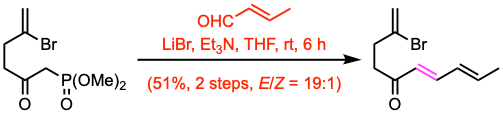
To a solution of anhydrous LiBr (1.20 equiv) in THF (100 mL) was added a solution of the crude substrate (44.2 mmol, 1.0 equiv) in THF (100 mL) via cannula at room temperature. After 10 min of stirring, Et3N (1.69 equiv) was added and the stirring was continued for 1 h. The mixture was cooled to 0 ºC and (E)-2-butenal (2.96 equiv) was added dropwise. After 6 h of stirring at room temperature, the mixture was quenched with sat. aq. NH4Cl and diluted with hexane/Et2O (1:1). The mixture was washed with brine, and the aqueous layer was extracted with hexane/Et2O (1:1). The extracts were combined, dried (Na2SO4), and concentrated in vacuo. The residue was purified by SiO2 flash column chromatography.
Example 2: J. Am. Chem. Soc. 2021, 143, 8261.
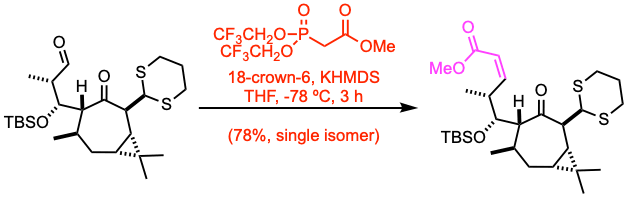
To a well stirred solution of 18-crown-6 (5.0 equiv) in THF (16 mL) at –78 °C was added 0.5M solution of KHMDS in PhMe (1.5 equiv) and the reaction was stirred for 20 minutes. After addition of the substrate (1.03 mmol, 1.0 equiv) and stirring for 3 hour the reaction was quenched with sat. aq. NH4Cl, extracted with diethyl ether, dried over Na2SO4 and concentrated in vacuo. Purification by flash column chromatography yielded the product (78% yield).
Example 1: J. Org. Chem. 2021, 86, 3074.

The substrate (1 mmol, 1.0 equiv) was dissolved in 1:1 THF/H2O (20 mL, 0.05 M), and potassium carbonate (4.5 equiv) was added. The mixture was stirred at room temperature for 2 h and was then diluted with saturated NH4Cl solution and ethyl acetate. The phases were separated, and the aqueous layer was extracted with EtOAc. The combined organic extracts were washed with brine, dried over anhydrous Na2SO4, and concentrated under reduced pressure to give a crude oil. Purification of the residue by flash chromatography afforded the desired alkene.
Videos about Horner-Wadsworth-Emmons reaction
Images of Horner-Wadsworth-Emmons reaction
Online database of named reactions
Browse named reactions in alphabetical order or by category in our online database of organic reactions.