The Hofmann rearrangement allows the conversion of primary carboxamides to the corresponding one-carbon shorter amines. There is complete retention of configuration when enantiopure amides are used.

- The reaction can form a wide range of products, including alkyl and arylamines.
- Amides cannot contain base-sensitive functional groups; acid-sensitive groups (e.g., acetals) may be present.
- The isocyanate intermediate is readily hydrolyzed under these reaction conditions; it can not be isolated.
- There is complete retention of configuration when enantiopure amides are used (see example 1).
- In the standard procedure, the reaction of bromine with aqueous sodium hydroxide forms sodium hypobromite in situ, which converts the primary amide into an intermediate isocyanate. This intermediate is then hydrolyzed to the corresponding primary amine. However, these conditions are not suitable for base-sensitive substrates. To accommodate base-sensitive substrates, the oxidative Hofmann rearrangement can be performed with lead tetraacetate (LTA) or hypervalent iodine reagents (such as PIDA or PIFA) under mildly acidic conditions (see example 1).
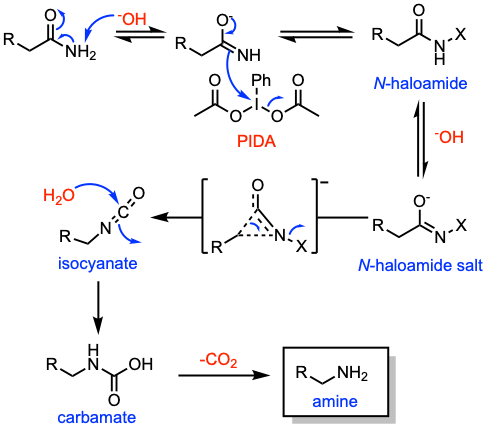
Reaction mechanism of Hofmann rearrangement
A simplified suggested mechanism is presented: (i) Formation of the N-halogen amide. (ii) Deprotonation of the N-haloamide by the base and formation of the alkali salt. (iii) Concerted rearrangement to the isocyanate via a bridged anion. (iv) Hydration and decarboxylation to afford the corresponding amine.
Examples and experimental procedures of Hofmann rearrangement
Example 1: J. Am. Chem. Soc. 2019, 141, 2233.

The amide (0.21 mmol, 1.0 equiv) was dissolved in 1,4-dioxane (4.1 mL), and H2O (4.1 mL) was added. To this solution, crushed KOH (35.0 equiv) was added, and the suspension was allowed to stir for 5 min at room temperature. At this point, PIDA (1.2 equiv) was added. The suspension was stirred at room temperature for 20 h, after which it was quenched with NaHCO3 and Na2S2O3. Following a 5-minute stirring period at room temperature, the mixture was diluted with EtOAc, and the aqueous layer was extracted with EtOAc. The combined organic layers were washed with brine, dried over Na2SO4, and concentrated under vacuum to yield the crude reaction product. The crude product was purified by preparatory thin-layer chromatography to afford the primary amine.
Videos about Hofmann rearrangement
Images of Hofmann rearrangement
Online database of named reactions
Browse named reactions in alphabetical order or by category in our online database of organic reactions.