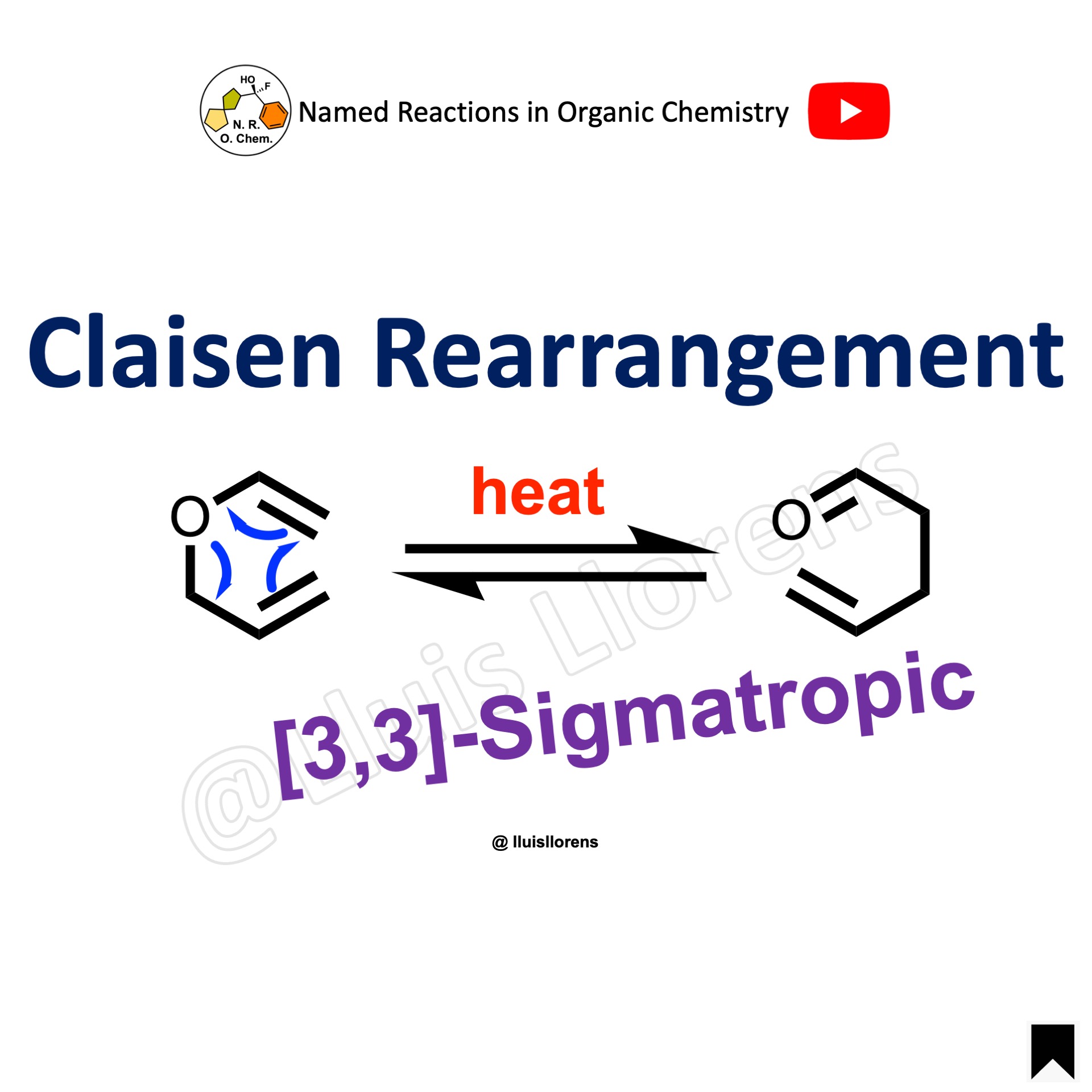
The Claisen rearrangement is a pericyclic reaction involving a concerted mechanism with a cyclic transition state. Specifically, it belongs to the group of sigmatropic reactions, where one σ-bond is exchanged for another. In the Claisen rearrangement, a [3,3]-sigmatropic shift occurs, transforming an allyl vinyl ether into an γ,δ-unsaturated carbonyl compound.
- The Claisen rearrangement was originally applied to rearrangements of allyl aryl ethers to afford ortho– and occasionally para-substituted phenols. Afterward it expanded to analogue rearrangements of allyl vinyl ethers into unsaturated carbonyl compounds, which were classified as [3,3]-sigmatropic rearrangements.
- The Claisen rearrangement, as in any other [3,3]-sigmatropic rearrangement, takes place under thermodynamic control. This reaction is irreversible toward the formation of the carbonyl compounds due to their higher thermodynamic stability. In some cases, structural features leading to the relief in torsional strain shift the equilibrium toward the retro-Claisen isomer, favoring the transformation of the carbonyl compound into the vinyl ether.
- This transformation can be chemo-, regio-, diastereo-, and enantioselective; the reaction can be performed under mild conditions, and affords potentially useful polyfunctionalized molecules.
- The most frequently reported Claisen rearrangement is a process that requires high temperatures (see example #1) and proceeds quite slowly at atmospheric pressure.
Reaction mechanism of Claisen rearrangement
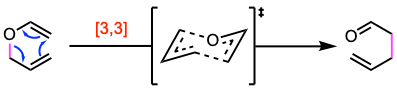
The reaction mechanism involves a concerted rearrangement through a chair-like transition state. However, there is no general agreement about the structure of this transition state. Different transition states (synchronic, fragmented, or 1,4-diyl) have been proposed.
For more details, refer to Chem. Rev. 2004, 104, 2939.

In the Claisen rearrangement of an allyl aryl ether, the first [3,3] step affords an ortho dienone which usually enolizes into an o-allylphenol. It is the reaction known as the ortho-Claisen rearrangement.
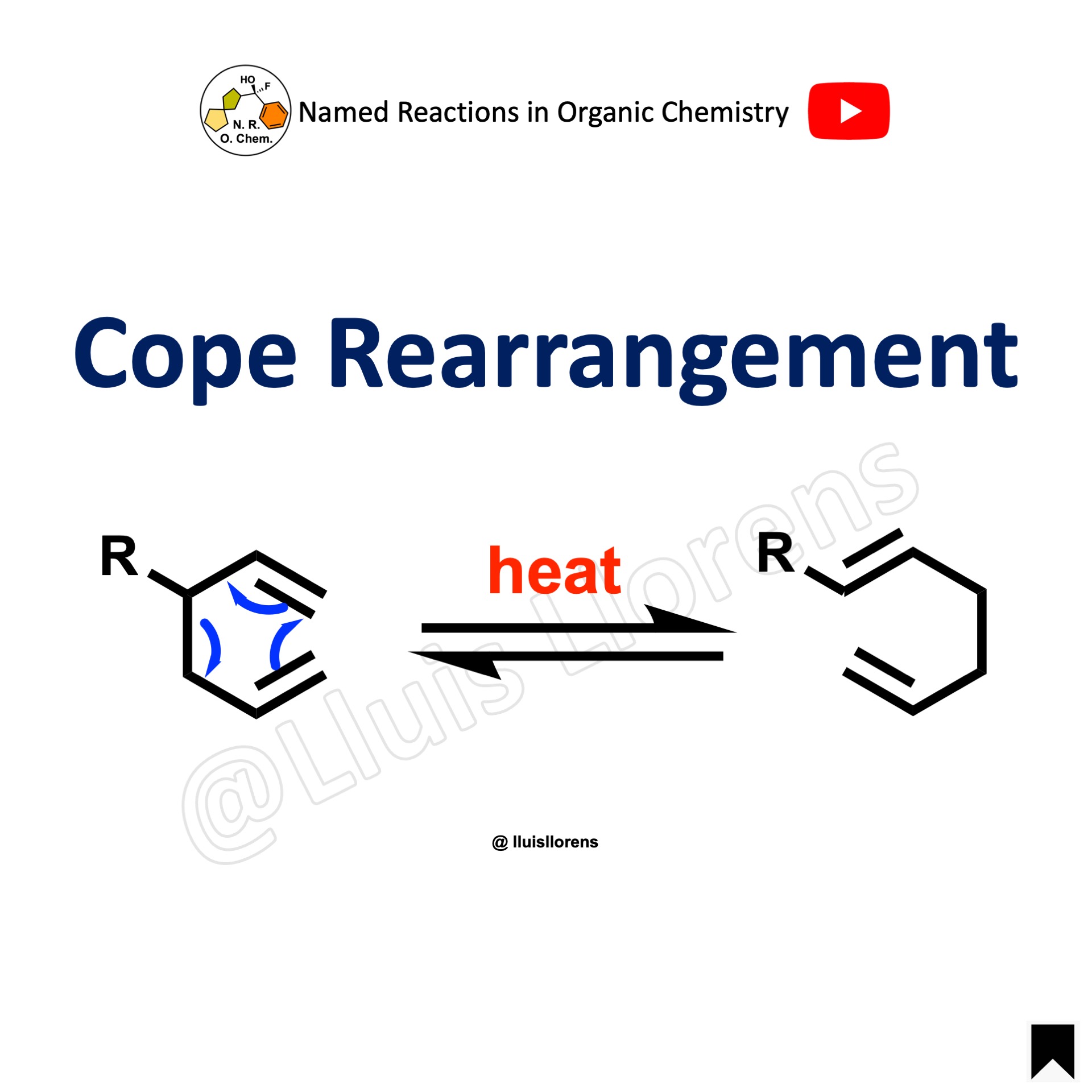
When the rearrangement takes place on an ortho position bearing a substituent, a second [3,3] rearrangement (Cope rearrangement) takes place followed by enolization.
This reaction, usually called the para Claisen rearrangement, leads to the corresponding p-allylphenol.
The product resulting from the ortho Claisen rearrangement is usually obtained from the reaction, although the para process can compete even when both ortho positions are not occupied.

If the substrate is a chiral molecule due to the presence of a substituent (e.g., iPr) of the allyl vinyl ether, particularly in the case of acyclic molecules, this chirality can be transferred to the 1 and/or 6 positions through a cyclic transition state.
Examples and experimental procedures of Claisen rearrangement
Example 1: J. Am. Chem. Soc. 2021, 143, 4379.

A solution of the allyl vinyl ether (30.5 mmol, 1.0 equiv) in DMF (250 mL) was heated at 140–150 ºC for 18 h. Upon completion, the reaction mixture was cooled to room temperature, diluted with Et2O, and washed with sat. aq. NaHCO3 and water. The combined aqueous layers were further extracted with Et2O. The combined organic layers were dried over Na2SO4, filtered, and concentrated. The residue was purified by flash column chromatography to afford the unsaturated carbonyl product, which was obtained as a single diastereomer.
Videos about Claisen rearrangement
Images of Claisen rearrangement
Online database of named reactions
Browse named reactions in alphabetical order or by category in our online database of organic reactions.