The Buchwald-Hartwig coupling is a direct Pd-catalyzed C–N or C–O bond formation reaction between aryl halides and amines or alcohols, typically conducted in the presence of a stoichiometric amount of base.
- The coupling can be both intermolecular and intramolecular (see example 3).
- The typical substrates for this transformation are aryl bromides or iodides, but aryl chlorides can also be coupled with the desired amines or alcohols (see example 4).
- The Pd(0)-catalyst is usually complexed with chelating phosphine-type ligands such as BINAP (see example 4), dppf, Xantphos (see example 2), BrettPhos (see example 3), etc.
- Monoarylation of ammonia is difficult as primary (hetero)aryl amines readily undergo secondary arylation. Using ammonia surrogates like benzophenone imine prevents this, yielding a protected product that may be transformed into the desired amine (see example 4).
- The base has to be present in stoichiometric amounts and the temperature for the reaction can be sometimes as low as 25 °C. For more details, see J. Org. Chem. 2014, 79, 11961.
- For base-sensitive five-membered heteroaryl halides, see J. Am. Chem. Soc. 2023, 145, 3323.
- For hindered secondary amines, see Angew. Chem. Int. Ed. 2015, 54, 8259.
- For insights into the selection of reaction conditions, see Tetrahedron 2019, 75, 4199 and Chem. Sci. 2011, 2, 27.
- For the asymmetric Buchwald-Hartwig coupling, see Angew. Chem. Int. Ed. 2023, 62, e202216863.
Reaction mechanism of Buchwald-Hartwig coupling
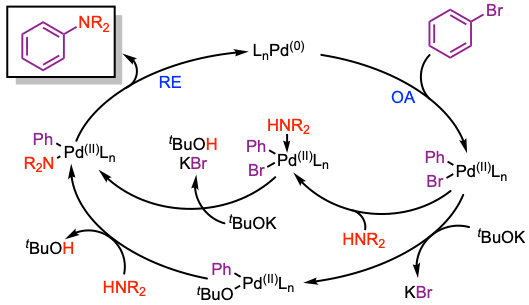
1. Oxidative addition (OA) of Pd(0) to the aryl halide.
2. The Pd(II)-aryl amine is either formed by direct displacement of the halide by the amine or via a Pd(II)-alkoxide intermediate.
3. Reductive elimination (RE) results in the formation of the C–N bond and the regeneration of the Pd(0) catalyst.
For more details see, J. Organomet. Chem. 2018, 861, 17, Tetrahedron 2019, 75, 4199, and Angew. Chem. Int. Ed. 2019, 58, 17118.
- Oxidative addition: In terms of the aryl electrophile, the general order of reactivity is ArI > ArBr ∼ ArOTf > ArCl ∼ ArOMs. Using this hierarchy, it is often possible to chemoselectively couple one electrophilic site, leaving another available for subsequent functionalization.
- In many cases the rate of transmetalation is fastest for aryl chlorides (within the halide series), due to the increased polarity of the Pd–Cl bond, and smaller size of Cl relative to Br and I. Contrary to conventional wisdom, the use of aryl iodide substrates should be avoided if possible. Aryl iodides can be challenging substrates, as the NaI formed during the reaction may have an inhibitory effect. Using a solvent that does not solubilize NaI (e.g., toluene) can help mitigate this issue.
- In general, reductive elimination is a rapid process in most C–N cross-coupling reactions. Reductive elimination is more challenging for less nucleophilic coupling partners, such as diarylamines. This step can be facilitated by withdrawing electron density from the metal center through the use of electron-poor phosphine ligands (e.g., JackiePhos).
Examples and experimental procedures of Buchwald-Hartwig couplings
Example 4: J. Med. Chem. 2021, 64, 3604.
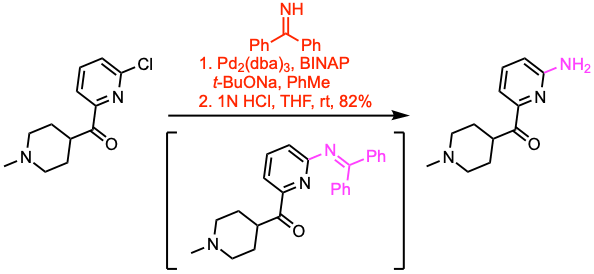
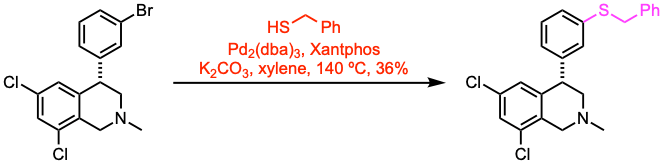
Example 3: J. Am. Chem. Soc. 2023, 145, 25533. Open access.

A glass vial containing the amine (2.64 mmol, 1.0 equiv) was brought into a nitrogen-filled glovebox. To this vial were added BrettPhos Pd G4 (5 mol%), BrettPhos (5 mol%), and K3PO4 (1.4 equiv), followed by t-BuOH (6.6 mL). The vial was sealed with a PTFE-lined cap and electrical tape, removed from the glovebox, and stirred at 100 ºC in a metal heating block. After 3 days, the reaction mixture was partitioned between DCM and water, and the layers were separated. The aqueous phase was extracted with DCM, and the combined organic phases were concentrated under reduced pressure. The crude product was purified by flash column chromatography.
Example 2: J. Med. Chem. 2021, 64, 3604.
Example 1: J. Org. Chem. 2021, 86, 7773.
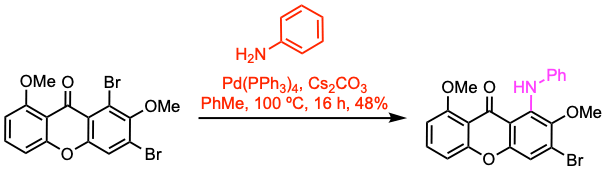
A screwcap vial equipped with a magnetic stir bar was charged with the aryl halide (0.13 mmol, 1.0 equiv), Pd(PPh3)4 (0.05 equiv), and Cs2CO3 (2.0 equiv). A solution of freshly distilled aniline (2.0 equiv) in anhydrous PhMe (1.30 mL) was added, and the cap was fitted. The contents of the vial were stirred at 100 oC for 16 h. The reaction mixture was allowed to cool to room temperature, the vial was carefully opened, and the contents were partitioned between EtOAc and H2O. The aqueous phase was extracted with EtOAc, and the combined organic phases were washed with brine, dried over Na2SO4, and concentrated under vacuum. The residue was purified by flash column chromatography to give the secondary amine.
Videos about Buchwald-Hartwig coupling
Images of Buchwald-Hartwig coupling
Online database of named reactions
Browse named reactions in alphabetical order or by category in our online database of organic reactions.