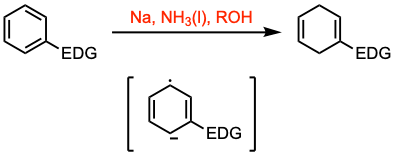
The Birch reduction involves the 1,4-reduction of aromatic rings to produce unconjugated cyclohexadienes and heterocycles. This transformation is achieved using alkali metals (Na, Li, K) dissolved in liquid ammonia in the presence of an alcohol, which serves as a proton donor. The regioselectivity of the reduction is influenced by the nature of the substituent on the aromatic ring.
- The empirical rule is that for electron donor substituents/groups (EDG) such as methoxy or methyl the dihydro aromatic is formed with the maximum number of substituents on the residual double bonds (see example 1 and example 2). With electron withdrawing groups (EWG), the rule is reversed.
- Experimentally, the reaction is run in liquid ammonia with an alkali metal and an added alcohol. Two representative examples are the reaction of anisole (with an EDG) and that of benzoic acid (with an EWG).
- The rate of the reduction is lower in the presence of electron-donating substituents.
- For a superior method for reducing phenol ethers to dihydro derivatives and unsaturated ketones, see J. Am. Chem. Soc. 1953, 75, 5360.
- For a scalable Birch reduction with lithium and ethylenediamine in tetrahydrofuran, see: Science 2021, 374, 741.
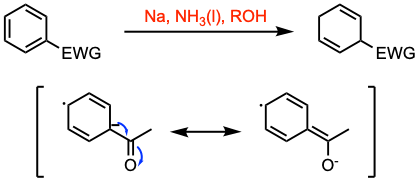
Reaction mechanism of Birch reduction

1. The solution of the metal in ammonia provides electrons, which are taken up by the aromatic ring to form the corresponding radical anion.
2. Protonation by the alcohol generates a cyclohexadienyl radical.
3. Formation of a cyclohexadienyl carbanion occurs by electron transfer.
4. Finally, protonation leads to the formation of the unconjugated cyclohexadiene product.

The reaction involves an initial radical anion resulting from the introduction of an electron from the blue liquid ammonia solution of free electrons formed by the dissolution of Li or related metals. This radical anion is protonated by an alcohol and then further reduced to a carbanion. Finally, the carbanion is protonated using a second proton to afford a nonconjugated cyclohexadiene.
- The regiochemistry depends on the substituents present.
- The rate-limiting step is the protonation of the radical-anion to afford the radical intermediate.
- For additional details on the Birch reduction mechanism, refer to Acc. Chem. Res. 2012, 45, 164.
Examples and experimental procedures of Birch reduction
Example 2: Org. Lett. 2024, 26, 2893. Open access.
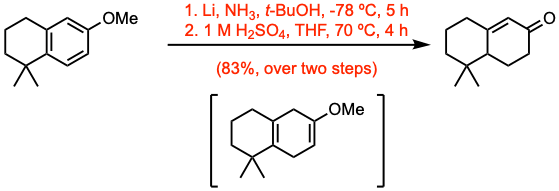
In a three-necked round-bottom flask was prepared a solution of lithium (5.0 equiv) in liquid ammonia (130 mL) at -78 ºC. Then, a solution of the substrate (33.5 mmol, 1.0 equiv) in THF (7.0 mL) was added. After 20 min, t-BuOH (2.4 equiv) was added. Stirring was continued for 5 h at this temperature until the reaction was quenched by the addition of sat. aq. NH4Cl. The mixture was allowed to warm to room temperature overnight, to evaporate the remaining ammonia. The resulting slurry was then taken up in MTBE, filtered and concentrated under reduced pressure. The crude diene was then dissolved in THF (112 mL), and aq. H2SO4 (1.0 M, 120 mL) was added. The reaction mixture was stirred at reflux (oil bath) for 4 h. The aqueous phase was extracted with MTBE three times, and the combined organic phases were washed with sat. aq. NaHCO3 and brine. After drying over MgSO4, filtration, and concentration under reduced pressure, flash column chromatography gave the desired enone.
Example 1: Angew. Chem. Int. Ed. 2021, 60, 12392.

To a solution of p-methoxyphenethyl alcohol (65.7 mmol, 1.0 equiv) and tert-butanol (20 mL) in liquid NH3 at -78 ºC, Li (6.0 equiv) was added portionwise. After 5 h at this temperature, the reaction mixture was slowly quenched with NH4Cl (8.0 equiv), and the cooling bath was removed. The mixture was stirred overnight at room temperature with a flow of nitrogen to evaporate the residual NH3, and water was added. The product was then extracted with MTBE. The combined organic phases were dried over MgSO4 and concentrated under reduced pressure to obtain the desired product.
Video about Birch reduction
Images of Birch reduction
Online database of named reactions
Browse named reactions in alphabetical order or by category in our online database of organic reactions.