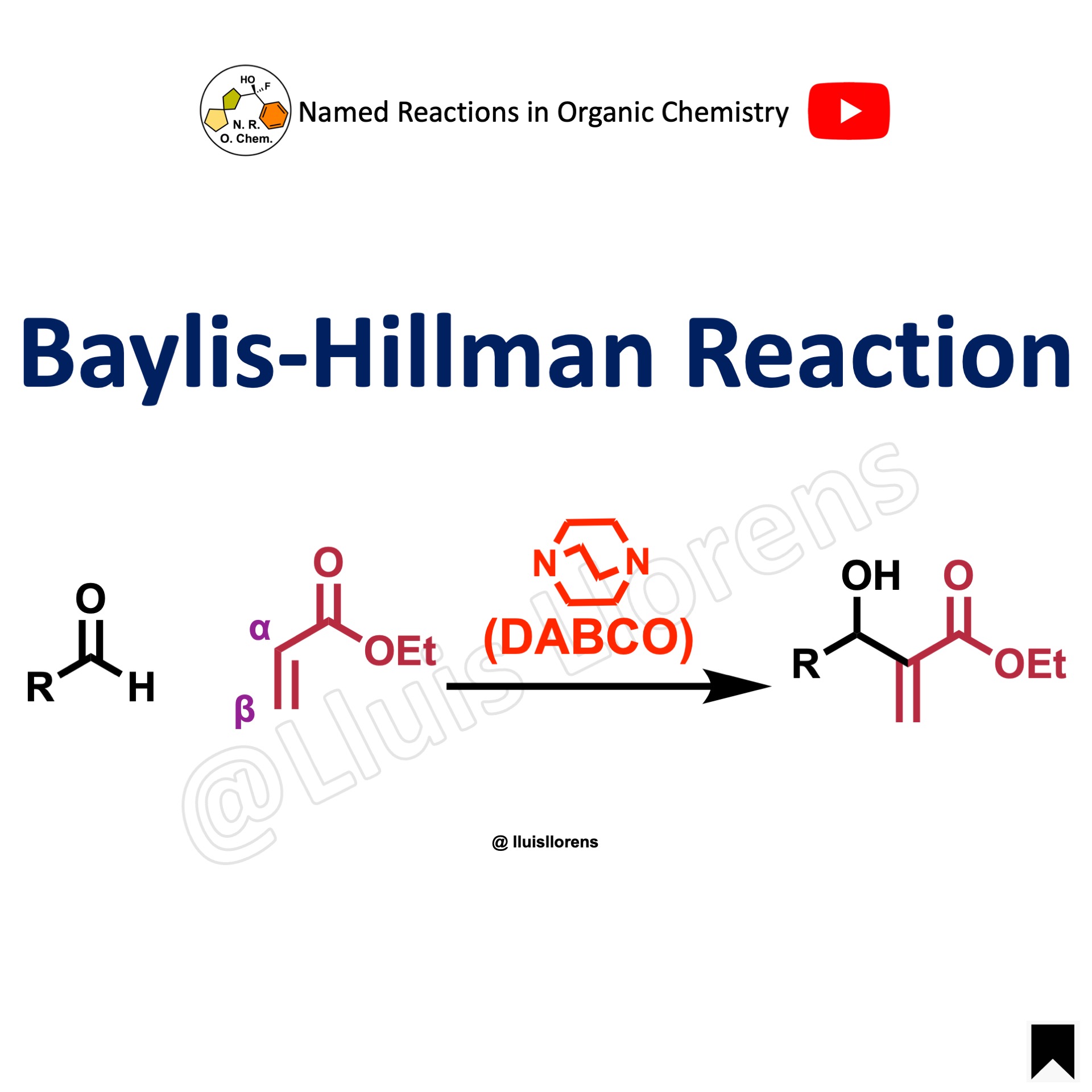
The Corey-Bakshi-Shibata (CBS) reduction allows the enantioselective reduction of ketones using oxazaborolidine catalysts; it is also known as the Corey-Itsuno reduction.
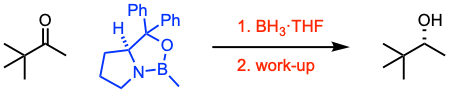
- When the oxazaborolidine has a rigid bicyclic structure, high enantiomeric excess can be achieved.
- The CBS catalyst presents good air and moisture stability. However, the presence of water in the reaction mixture has been shown to have a significant effect on enantiomeric excesses. Hence, the reduction must be conducted under anhydrous conditions.
- This reaction is an effective and powerful method to reduce a wide range of ketones in a stereoselective and chemoselective manner.
- The driving force for the face-selective intramolecular hydride transfer has two main reasons: (i) the activation of the borane reagent by coordination to the Lewis basic nitrogen and (ii) the enhancement of the Lewis acidity of the boron atom (of the catalyst) for coordination to the ketone.
- Reductants range from relatively Lewis acidic BH3·THF and BH3·SMe2 complexes to catecholborane to complexes between amines and boranes.
Reaction mechanism of Corey-Bakshi-Shibata reduction
The reaction involves the Lewis base activation of the reductant and the Lewis acid activation of the electrophilic substrate. An interesting aspect of the activation of the electrophilic substrate is the enhanced Lewis acid character of the endocyclic boron. This feature is partially responsible for the enhanced rate of the catalytic CBS reduction over the background hydroboration reaction. The active reductant is the complex between the Lewis basic pyrrolidine nitrogen atom of the CBS catalyst and the stoichiometric borane reductant. For more details on the reaction mechanism of the CBS reduction, see Angew. Chem. Int. Ed. 2021, 133, 4873 (open access) and Lewis Base Catalysis in Organic Synthesis (2016): 387-456.
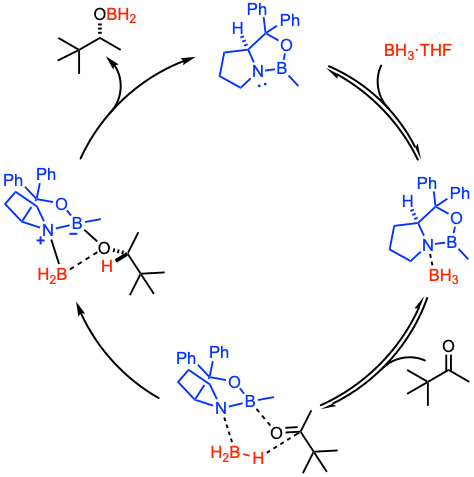
(i) Coordination of BH3 (Lewis acid) to the tertiary nitrogen atom (Lewis base) of the CBS catalyst to enhance the Lewis acidity of the boron atom and to activate the BH3 to become a strong hydride donor. (ii) The CBS catalyst-borane complex then binds to the ketone at the sterically more accessible lone pair, the lone pair closer to the smaller substituent. (iii) Hydride transfer via a six-membered transition state delivers the alkoxyborane and regenerates the catalyst. (iv) Final acidic workup yields the desired chiral alcohol.
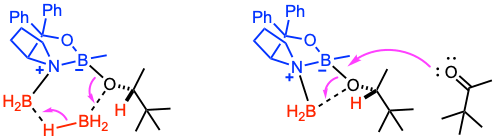
A simple four-center dissociation of the borinate ester of the resulting alkoxide or borane-mediated regeneration of the active catalyst–reductant complex are two possibilities when considering BH3 as the stoichiometric reductant. A third possibility is that the Lewis basic nitrogen atom can complex with the borinate ester of the product to effect a second reduction. It is possible that all three of these routes occur to different extents, depending on the Lewis basicity of the oxygen residing on the carbonyl substrate and the Lewis acidity of the stoichiometric reductant.
Examples and experimental procedures of Corey-Bakshi-Shibata reduction
Example 2: J. Am. Chem. Soc. 2022, 144, 5253.
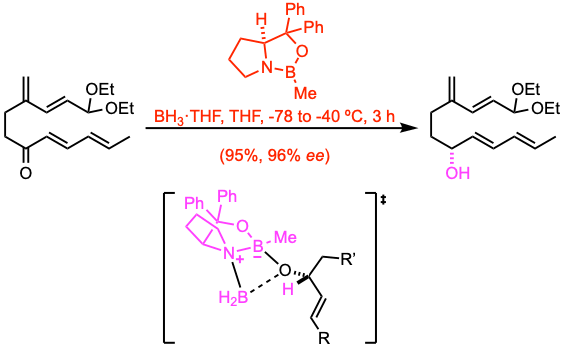
To a stirred solution of (S)-Me-CBS-oxazaborolidine (2.01 equiv) in THF (14 mL), BH3·THF (1.0 M in THF, 1.5 equiv) was added dropwise at 0 ºC. After 15 min of stirring at the same temperature, a solution of the substrate (5.28 mmol, 1.0 equiv; azeotropically dried with toluene) in THF (29 mL) was added dropwise at -78 ºC, and the resulting mixture was stirred for 1 h at the same temperature. BH3·THF (1.0 M in THF, 1.5 equiv) was then added dropwise over 1 h at -78 ºC. The mixture was gradually warmed to -40 ºC over 30 min, and stirring was continued for 30 min at the same temperature. The mixture was quenched with sat. aq. NH4Cl and diluted with CHCl3. The resulting mixture was washed with brine, dried over Na2SO4, and concentrated under vacuum. The residue was purified by flash column chromatography to afford the secondary alcohol.
Example 1: Angew. Chem. Int. Ed. 2020, 59, 16475.
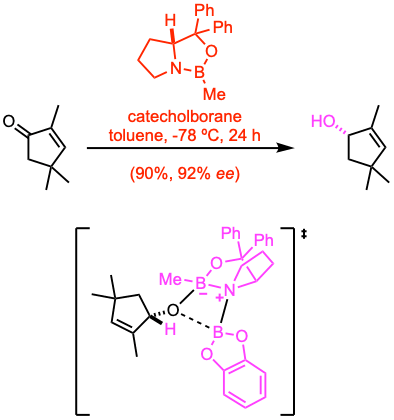
A solution of the cyclopentenone (56 mmol, 1.0 equiv) in anhydrous toluene (110 mL) was cooled to -78 ºC. (R)-2-methyl-CBS- oxazaborolidine (1.0 M in toluene, 0.2 equiv) was added, and the reaction mixture was stirred for 5 min at -78 ºC. Then, a solution of catecholborane (1.0 M in THF, 1.8 equiv) was added slowly, and the reaction mixture was stirred for 24 h at -78 ºC before it was quenched with sat. aq. NaHCO3. The resulting mixture was extracted with Et2O, and the combined organic extracts were sequentially washed with 2.0 M NaOH and brine, dried over Na2SO4, filtered, and concentrated under reduced pressure. The crude product was purified by flash column chromatography to afford the alcohol.
Video about Corey-Bakshi-Shibata reduction
Images of Corey-Bakshi-Shibata reduction
Online database of named reactions
Browse named reactions in alphabetical order or by category in our online database of organic reactions.