The Chan-Lam coupling is the cross-coupling reaction between an aryl boronic acid and an amine or alcohol to generate the corresponding secondary aryl amine or ether.

- In contrast to other cross-coupling reactions, the Chan-Lam reaction is unique in that it couples two nucleophiles: an aryl organoboron compound and an amine (see example 2) or alcohol (see example 1 and example 3).
- Typical substrates: phenols, amines, anilines, amides, imides, ureas, carbamates, sulfonamides, sulfoximes, and NH-heterocycles.
- Cu source: Cu(OAc)2, Cu(OTf)2, Cu(OPiv)2, Cu(acac)2, Cu(TFA)2, CuBr2, CuCl2, CuSO4, CuFAP, [Cu(DMAP)4I]I, [Cu(OH)·TMEDA]2Cl2, Cu(MeCN)4PF6, CuCl, Cu2O, Cu2S.
- Oxidant: O2(air), O2, pyridine N-oxide, TEMPO, (t-BuO)2.
- Base: Et3N, (i-Pr)2NEt, pyridine, 4-methylpyridine, 2,6-lutidine, K2CO3, K3PO4, KOt-Bu, N-methylpiperidine, n-Bu4NOH, NaOSiMe3, none.
- Solvents used: CH2Cl2, MeCN, EtOAc, MeOH, EtOH, 1,4-dioxane, NMP, THF, DMF, PhMe, DMSO, H2O, t-BuOH.
- Temperature range: rt to 100 °C.
- For synthetic applications of the Chan-Lam coupling, see: Mol Divers 2019, 23, 215 and Adv. Synth. Catal. 2020, 362, 3311.
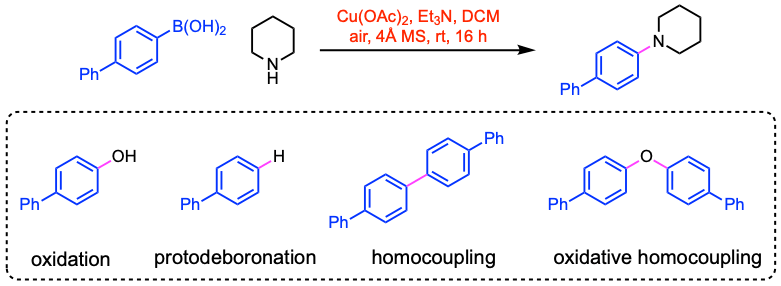
The utility of the Chan-Lam amination is often hampered by significant problems with by-product formation. Some by-products include the oxidation product, the protodeboronation product, or the homocoupling of the organoboron component.
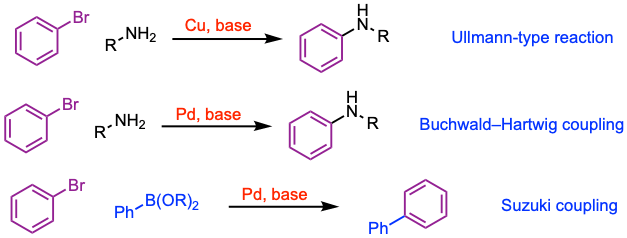
The Chan-Lam coupling can be viewed as a combination of the Suzuki coupling, which involves the aryl boronic component, and the Buchwald-Hartwig reaction, which involves the amine component. Unlike these reactions, which are catalyzed by palladium, the Chan-Lam coupling typically uses a catalytic or stoichiometric amount of copper, and the reaction can be conducted in air and at room temperature. It also bears resemblance to the Ullmann-type reaction, a copper-catalyzed nucleophilic aromatic substitution between nucleophiles such as amines and aryl halides. However, Ullmann-type couplings often require harsh conditions, such as high temperatures or strong bases.
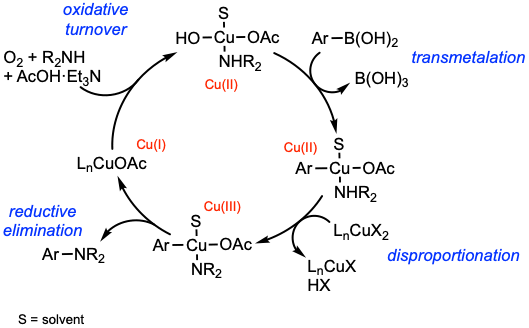
Reaction mechanism of Chan-Lam coupling
A suggested general mechanism of the Chan-Lam coupling is presented. Transmetalation of the organoboron compound, here a boronic acid, delivers a Cu(II) complex. Disproportionation using a second Cu(II) delivers the key Cu(III) complex. Reductive elimination forges the C–N bond, liberating the product and a Cu(I) species. Oxidative turnover of Cu(I) to Cu(II) using a terminal oxidant, most commonly O2, completes the catalytic cycle. For mechanistic aspects of the Chan-Lam coupling, see: Tetrahedron 2012, 68, 7735 and Chem. Rev. 2019, 119, 12491.
Examples and experimental procedures of Chan-Lam coupling
Example 3: Org. Lett. 2020, 22, 2022.

To a stirred solution of the boronic ester (10.9 mmol, 1.0 equiv) in DCM/MeOH (109 mL, 1:1) at room temperature, 4Å molecular sieves (activated, 6.0 g), Cu(OAc)2 (1.0 equiv), and DMAP (2.0 equiv) were added. The resulting mixture was stirred for 8 h before it was filtered through a pad of silica gel, eluted with EtOAc, and concentrated under reduced pressure. The resulting residue was purified by flash column chromatography to afford the coupled product.
Example 2: New J. Chem. 2020, 44, 308.
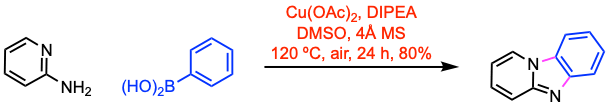
A 25 mL dry two-necked round bottom flask equipped with a magnetic stirrer and a guard tube containing calcium chloride was charged with 2-aminopyridine (1.0 mmol, 1.0 equiv), p-tolylboronic acid (2.0 equiv), DIPEA (3.0 equiv) and Cu(OAc)2 (1.0 equiv) in anhydrous DMSO (3 mL) with molecular sieves (200 mg). The mixture was heated at 120 ºC for 24 h. An aqueous solution of ammonium hydroxide was added, and the mixture was extracted with ethyl acetate. The organic layer was washed with brine, and dried over anhydrous sodium sulfate. After removal of the solvent under reduced pressure, the residue was purified by flash column chromatography.
Example 1: J. Org. Chem. 2018, 83, 3417.

To a 5 mL scintillation vial equipped with a Teflon-coated magnetic stir bar were added the phenol (0.3 mmol, 1.0 equiv), cyclopropyl potassium trifluoroborate (3.0 equiv), Cu(OAc)2 (10 mol%), 1,10-phenanthroline (10 mol%), and K2CO3 (2.0 equiv). The vial was charged with toluene (0.5 mL) and water (0.15 mL) and sealed with a septum cap. (Caution! Because the reaction involves heating an organic solvent under O2, a blast shield should be used at all times, particularly in large-scale experiments. After the indicated reaction time, the reaction vessel should be allowed to cool to room temperature behind the blast shield prior to workup.) The septum was pierced with a 21G needle connected to an O2 balloon. The septum cap was partially unscrewed to purge the vial for 5 seconds and then resealed by tightening the cap. The reaction was stirred under O2 (balloon) at 70 ºC for 12 h, after which it was quenched with sat. aq. NH4Cl and extracted with EtOAc. The combined organics were dried over MgSO4, filtered, and concentrated in vacuo. The crude product was then purified by flash column chromatography.
Videos about Chan-Lam coupling
Images of Chan-Lam coupling
Online database of named reactions
Browse named reactions in alphabetical order or by category in our online database of organic reactions.