The Babler-Dauben oxidation is the catalytic chromium-mediated 1,3-oxidative transposition of allylic alcohols to give α,β-unsaturated carbonyl compounds. Typical systems include the Jones reagent (CrO3/aq. H2SO4), pyridinium dichromate (PDC) and pyridinium chlorochromate (PCC).

- Controlling the oxidation of secondary allylic alcohols is more challenging compared to tertiary analogues, as the secondary alcohol can be readily oxidized to the corresponding ketone.
- The most common solvents for the Babler-Dauben oxidation are DCM and chloroform.
- The main drawback is the use of the highly toxic oxidizing agent, PCC.
- PDC has the advantage of producing carboxylic acids from primary alcohols when used in DMF. When used in dichloromethane, PDC performs very similarly to PCC in oxidizing secondary alcohols to ketones. The use of this milder, less-acidic oxidant is convenient for acid-sensitive substrates (see example 2).
- The focus on safer, less toxic alternatives to heavy-metal oxidants has led to the exploration of 2,2,6,6-tetramethylpiperidyl-1-oxyl (TEMPO). For the application of non-catalytic TEMPO as its oxoammonium salt in conjunction with BF4− counterion for the oxidative transposition of a tertiary allylic alcohol, see example 3.
Reaction mechanism of Babler-Dauben oxidation
A suggested mechanism of the Babler-Dauben oxidation. (i) The cyclic tertiary allylic alcohol adds to pyridinium chlorochromate, PCC, to form the corresponding chromate ester. (ii) 1,3-oxidative transposition affords the 3-alkyl α,β-unsaturated ketone. An ionization pathway in which the allylic alcohol is ionized, followed by the recombination of the carbocation with chromate, is also suggested. For more details, refer to Tetrahedron 2012, 68, 5323.

Examples and experimental procedures of Babler-Dauben oxidation
Example 3: Org. Lett. 2023, 25, 1941.

To a solution of the tertiary alcohol (a mixture of two isomers, 0.405 mmol, 1.0 equiv) in 1.4-dioxane (5 mL), TEMPO+BF4- (1.5 equiv) was added in one portion. After stirring for 3 h at room temperature, water was added to the mixture, and the volatiles were removed under reduced pressure. The aqueous phase was extracted with EtOAc. The combined organic phases were washed with brine, dried over anhydrous Na2SO4, filtered, and concentrated under reduced pressure. The residue was purified by flash column chromatography to afford the secondary alcohol. Note: more potent oxidation conditions (AZADOL, PIDA, 98%) were necessary to yield the enone.
Example 2: J. Am. Chem. Soc. 2022, 144, 12970.

Example 1: Angew. Chem. Int. Ed. 2021, 60, 14967.
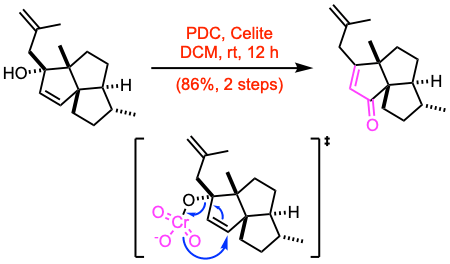
To a stirred solution of the crude allylic alcohol (19.2 mmol, 1.0 equiv) in dry DCM (300 mL) under argon, Celite (1.5 g per mmol of substrate) and finely ground PDC (3.0 equiv) were added. The suspension was stirred at room temperature in darkness for 12 h. After TLC indicated completion of the reaction, the mixture was filtered through a pad of silica gel and concentrated under reduced pressure to give a residue. This residue was then taken up with Et2O, filtered through a pad of silica gel, concentrated under reduced pressure, and finally purified by flash column chromatography to give the enone.
Video about Babler-Dauben oxidation
Images of Babler-Dauben oxidation
Online database of named reactions
Browse named reactions in alphabetical order or by category in our online database of organic reactions.