Tsuji-Trost Allylation
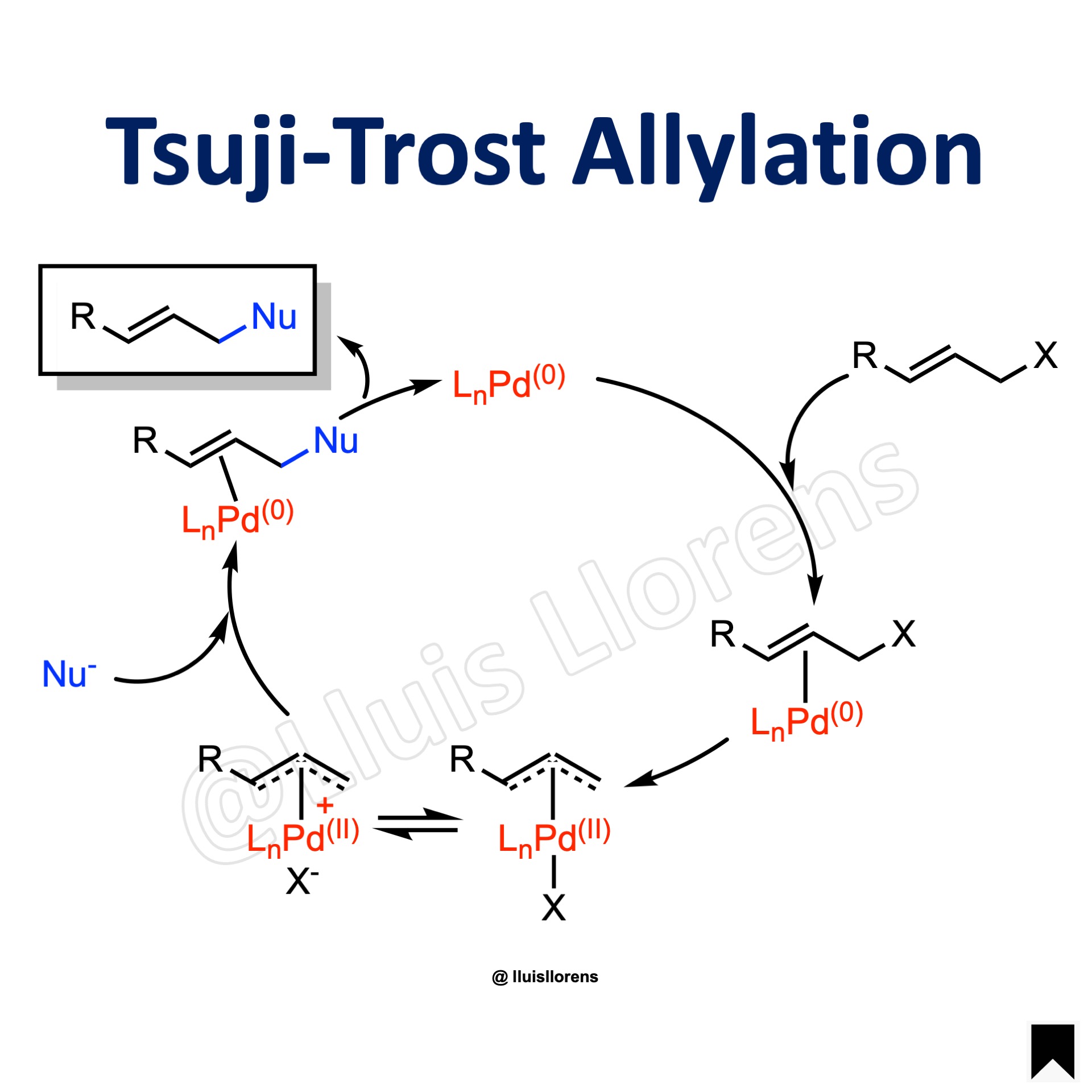
The Tsuji-Trost reaction is the palladium-catalyzed allylation of nucleophiles with allylic compounds via π-allylpalladium complexes.
General features:
1. The reaction is regioselective. And unsymmetrical allyl substrates usually undergo substitution at the least hindered allylic position. But the selectivity also depends on the size of the nucleophile and the nature of the ligands employed. 2. The oxidative addition goes with inversion of configuration. Then, depending on the strength of the nucleophile, the reaction can take two different pathways. Soft nucleophiles normally add directly to the allyl moiety, whereas hard nucleophiles attack first the metal center. 3. As a rule of thumb, soft nucleophiles are those substrates that typically have conjugate acids with pKas lower than 25, while hard nucleophiles typically have conjugate acids with pKas greater than 25.
Reaction Mechanism
1. The palladium catalyst coordinates with the allyl group, forming a π-allyl-Pd(0) complex. 2. Oxidative addition; the leaving group is expelled with inversion of configuration and a π-allyl-Pd(II) species is created. 3. The nucleophile adds to the allyl group regenerating the π-allyl-Pd(0) complex. 4. Finally, the palladium detaches from the alkene.
Experimental Procedure
To a suspension of t–BuOK (2.0 eq) in dry THF (160 mL) was added dimethyl malonate (2.2 eq) dropwise over 5 min at 0 °C under argon atmosphere. The yellow slurry was allowed to warm-up to 25 °C, and stirred at this temperature for 10 min. Pd(PPh3)4 (0.05 eq) was added in one portion. A solution of the allylic compound (49.5 mmol, 1.0 eq) in THF (40 mL) was added dropwise over 10 min. The reaction mixture was stirred at 50 °C for 12 hours. The mixture was quenched with water and extracted with EtOAc. The combined organic layers were washed with brine, dried over sodium sulfate, and concentrated. Purification by FCC afforded the desired compound (90% yield).
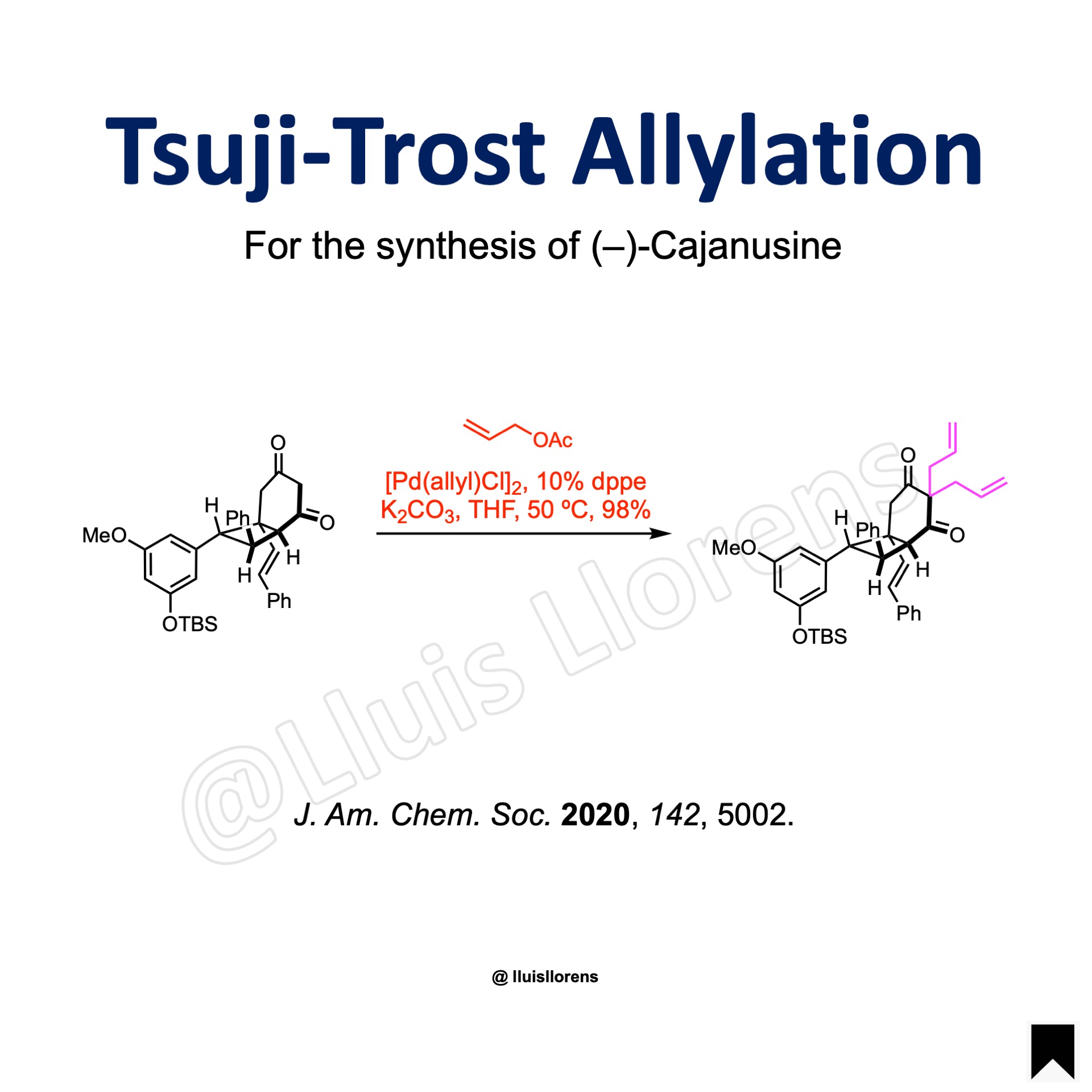
More Examples
Learn More Named Reactions
[instagram-feed feed=2]