Tebbe Olefination
The Tebbe olefination is the one-carbon homologation of carbonyl compounds using the Tebbe reagent.
General features:
1. The Tebbe reagent is commercially available, but it can be prepared from the reaction between titanocene dichloride with two equivalents of trimethylaluminum in toluene. This generates a methylene-bridged titanium-aluminum complexed that is often referred to as the Tebbe reagent. 2. The Tebbe reagent is more nucleophile and much less basic than the corresponding Wittig reagents. Therefore, less reactive carbonyl compounds can be readily olefinated, and it also presents more functional group tolerance. 3. Analogous to the Wittig reaction, the driving force is the high oxophilicity of the metal centre. 4. An alternative way to prepare the active olefinating reagent entails the use of the Petasis reagent, which can be prepared from the reaction between titanocenedichloride and methyl magnesium chloride or methyllithium. 5. In contrast to the Tebbe reagent, homologs of the Petasis reagent are relatively easy to prepare by using the corresponding alkyllithium reagents, allowing the conversion of carbonyl groups to alkylidenes. 6. Methylenation reactions occur for aldehydes and ketones as well as esters, lactones and amides. 7. The Tebbe reagent converts esters and lactones to enol ethers and amides to the corresponding enamines. 8. In compounds containing both ketone and ester groups, the ketone selectively reacts in the presence of one equivalent of the Tebbe reagent.
Reaction Mechanism
1. The Tebbe reagent is first exposed to a mild Lewis base, such as pyridine, to generate the active Schrock carbene. 2. The carbene reacts with the carbonyl compound to give an oxatitanacyclobutane intermediate. 3. The intermediate breaks down to the corresponding alkene.
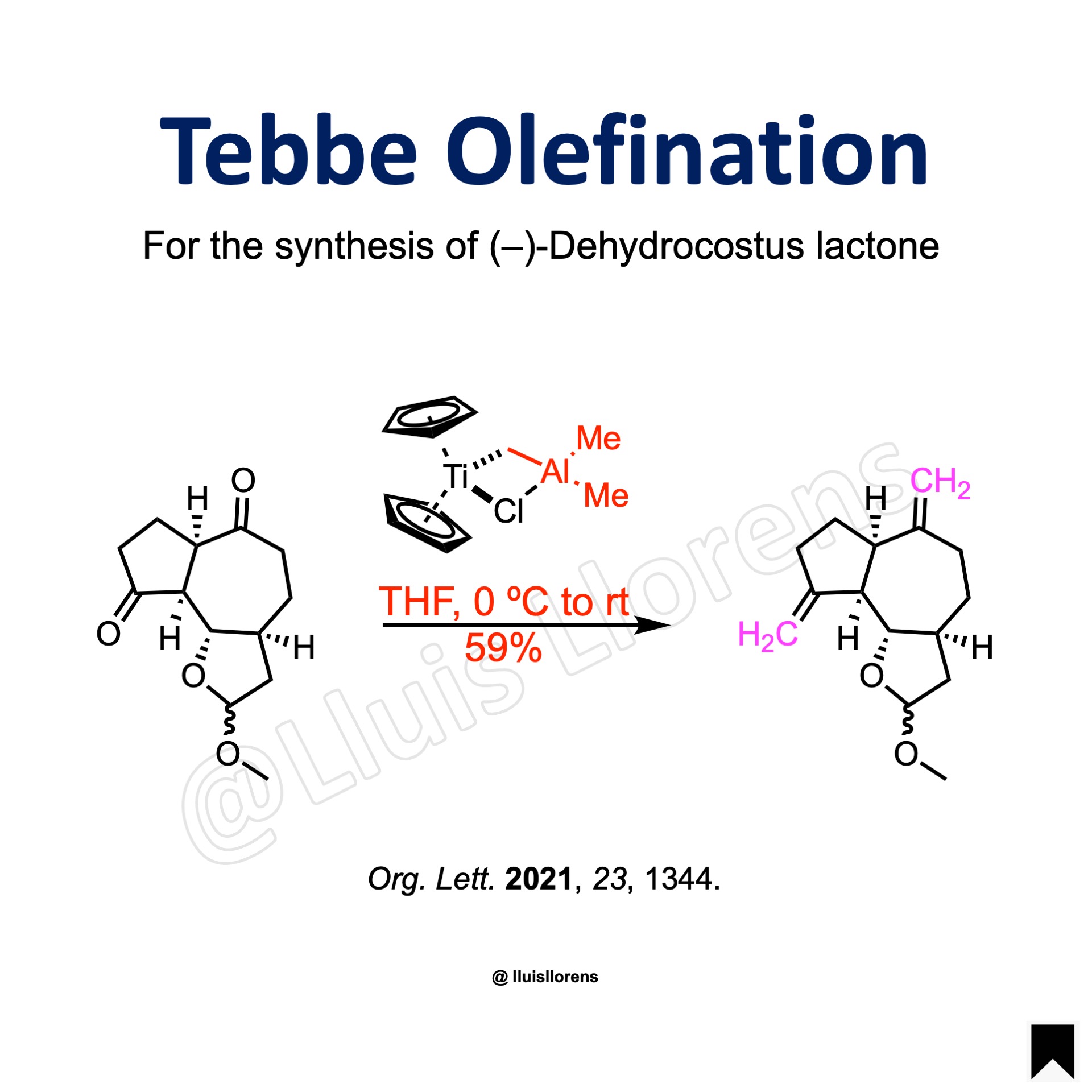
Example
Experimental Procedure
The diketone (67 µmol, 1.0 eq) was dissolved in THF (1 mL) and cooled to 0 °C. To this solution was added a solution of the Tebbe reagent (0.5 M in toluene, 3.0 eq). After warming to room temperature, the mixture was stirred for 30 min and subsequently diluted with Et2O. The reaction was quenched with aqueous NaOH. The mixture was dried over Na2SO4 and concentrated in vacuum. The residue was purified by flash column chromatography to give the one-carbon homologated product (59% yield).
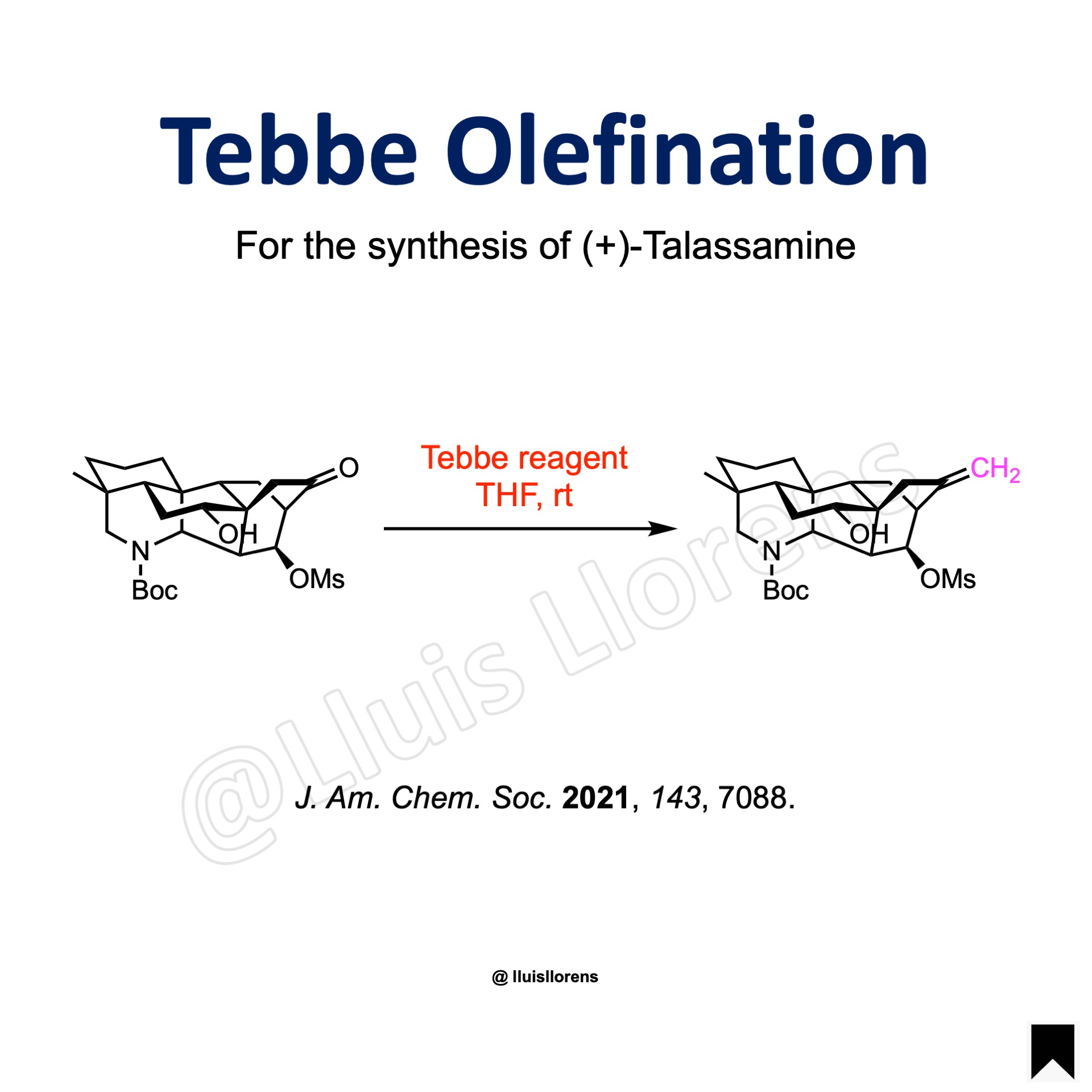
More Examples
Learn More Named Reactions
[instagram-feed feed=2]