Synthesis of Lascufloxacin
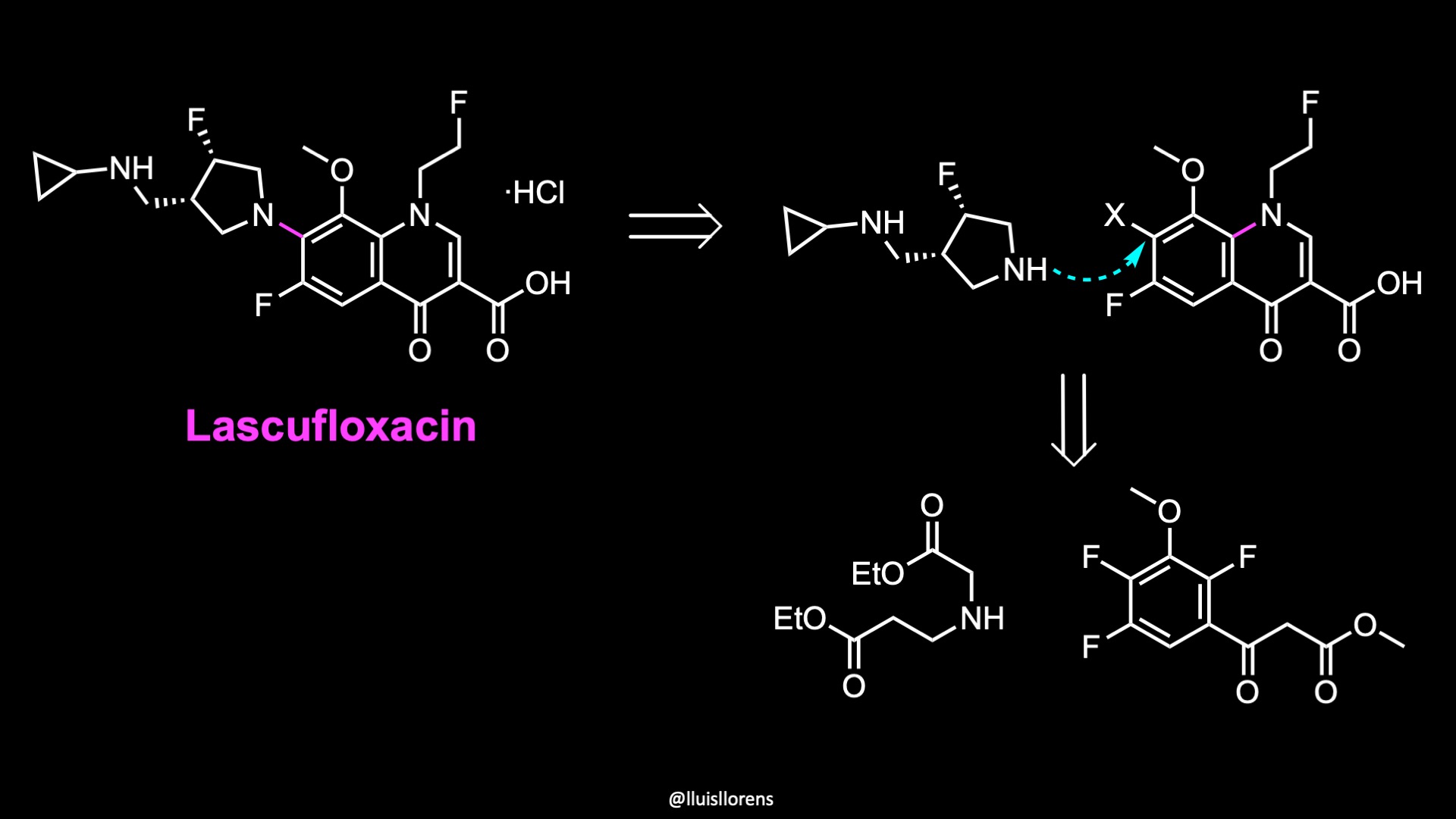
Retrosynthetic Analysis
The molecular structure consists of a fluoroquinolone core appended with a syn-disubstituted fluoropyrrolidine fragment. To prepare the fluoropyrrolidine fragment, the authors employed a linear route starting from this amino ester. The quinolone, on the other hand, was prepared from a commercial aryl oxopropanoate building block.
Synthesis steps
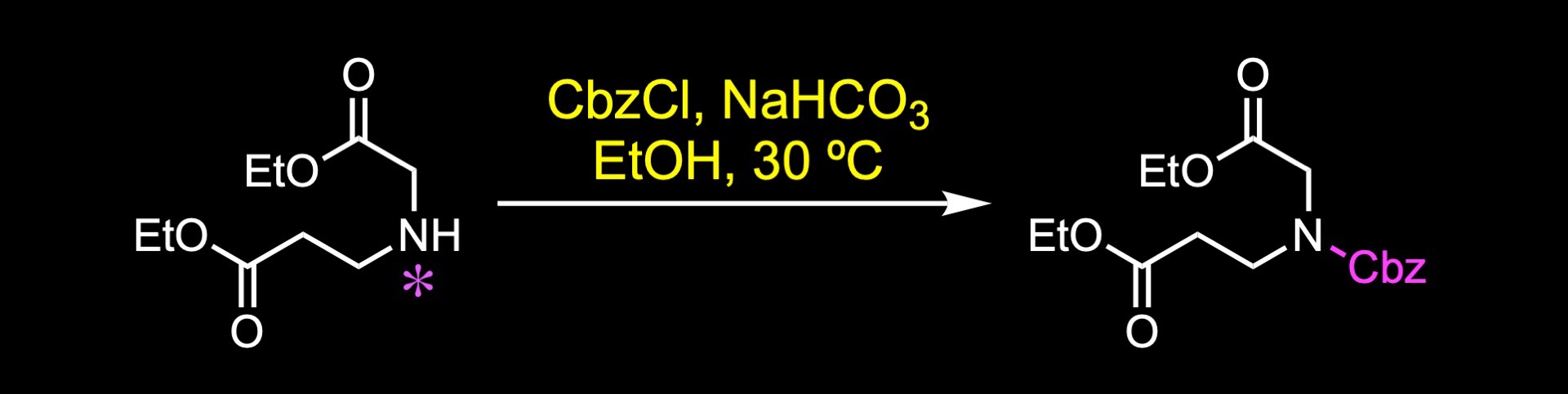
The synthesis commenced with Cbz protection of the secondary amine.
Then Claisen condensation delivered the corresponding pyrrolidone in 75% yield.
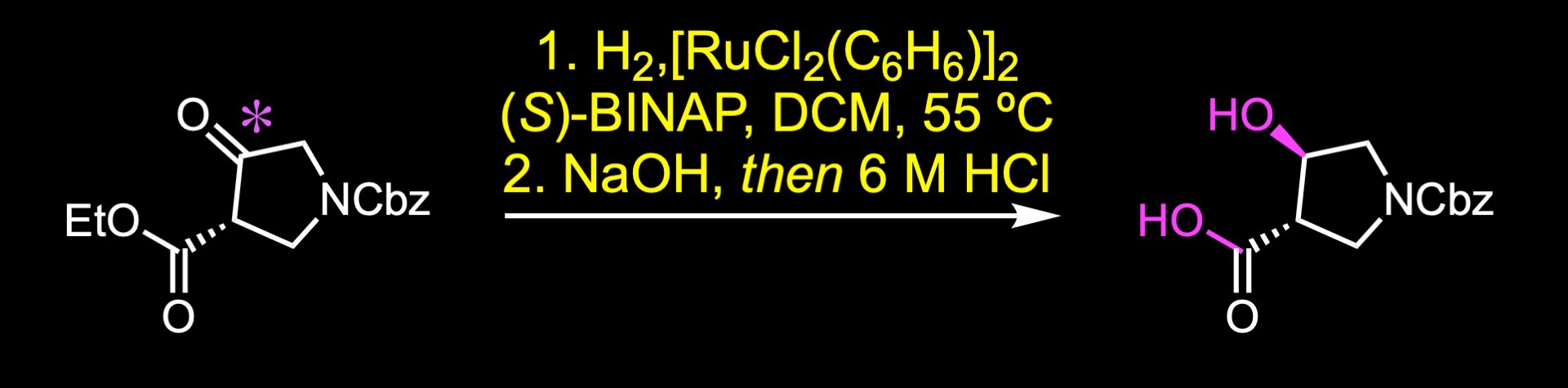
Asymmetric hydrogenation using a ruthenium catalyst with (S)-BINAP as the ligand provided this trans-pyrrolidine. Saponification of the resulting ester gave the corresponding carboxylic acid.
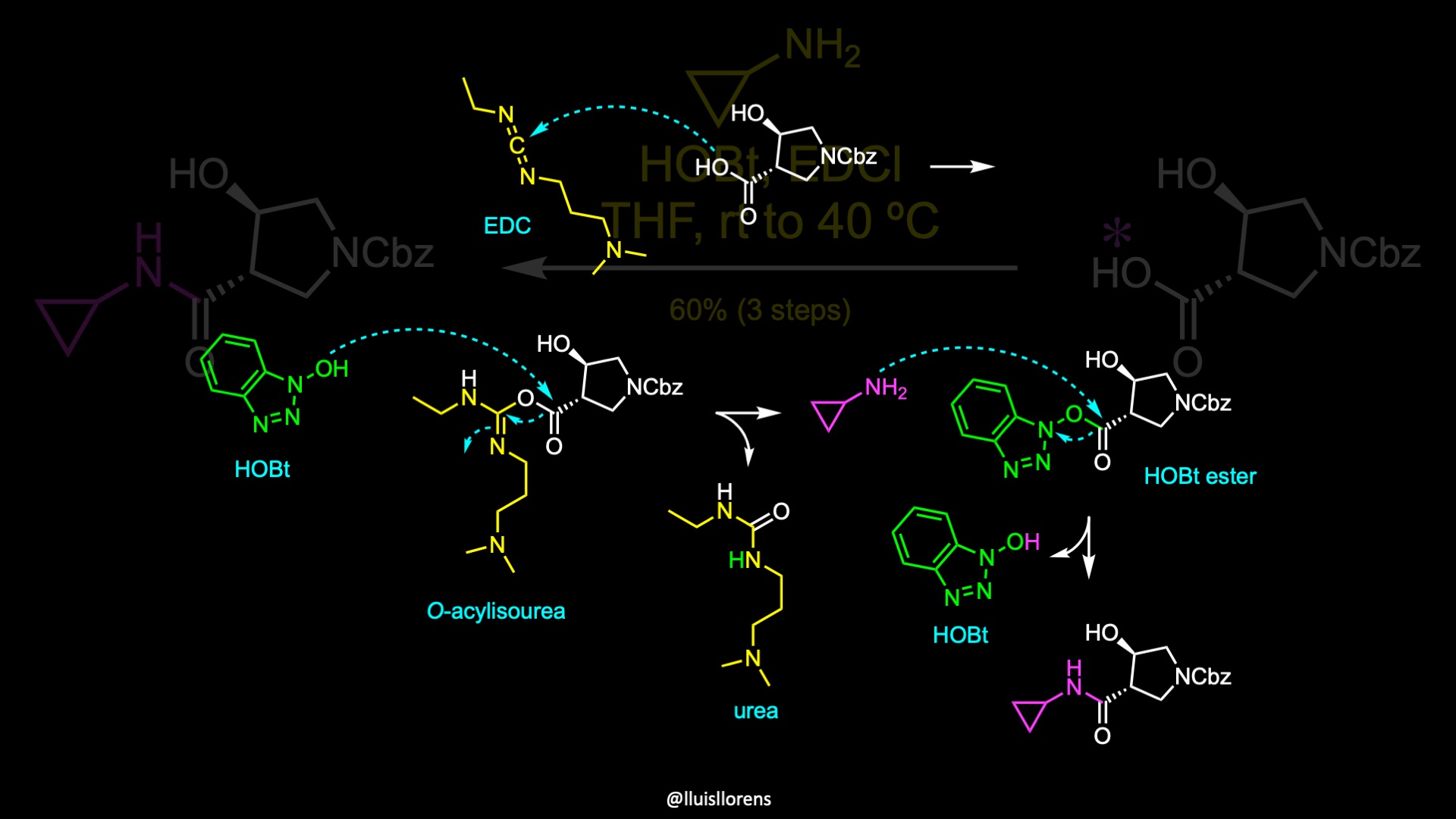
Coupling of this carboxylic acid with cyclopropylamine delivered a crystalline amide that was isolated in 60% yield in three steps.
In this amide coupling, the carboxylic acid is first activated by the carbodiimide to generate an O-acylisourea. Subsequent transesterification with hydroxybenzotriazole displaces the urea and delivers an active ester. The amine attacks then this ester, regenerating HOBt and delivering the desired coupled product.
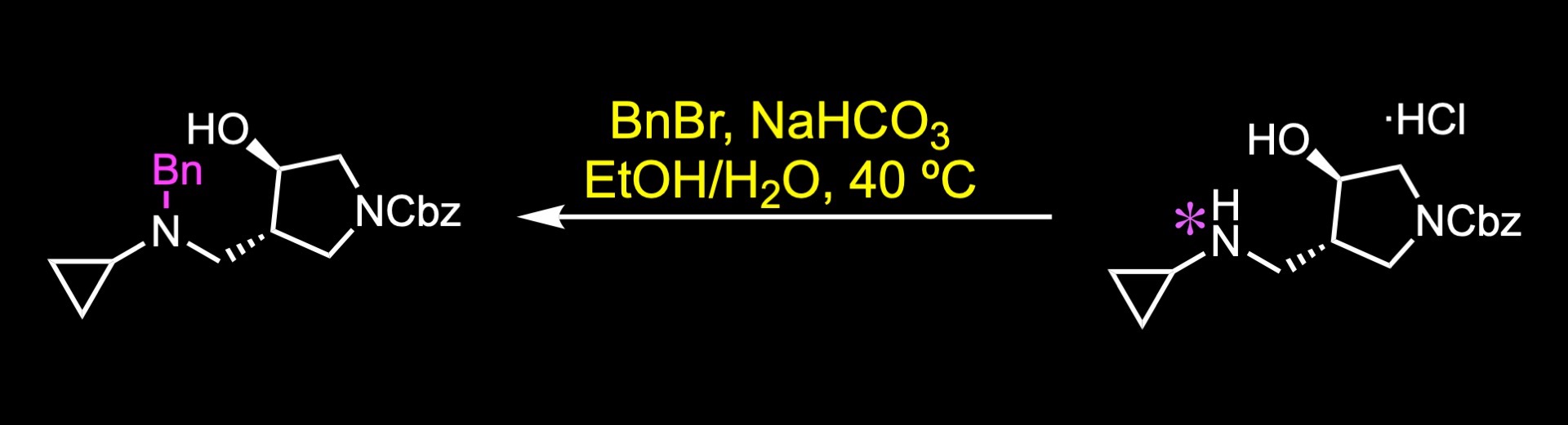
The amide was then reduced to the corresponding amine with borane in THF, and it was isolated as its hydrochloride salt.
Subsequent benzyl protection gave a trialkylamine that was treated with perfluoro-1-octanesulfonyl fluoride in the presence of DBU to deliver a chiral fluoride with inversion of stereochemistry.
Finally, removal of the protecting groups and salt formation produced the fluoropyrrolidine fragment as a crystalline solid.
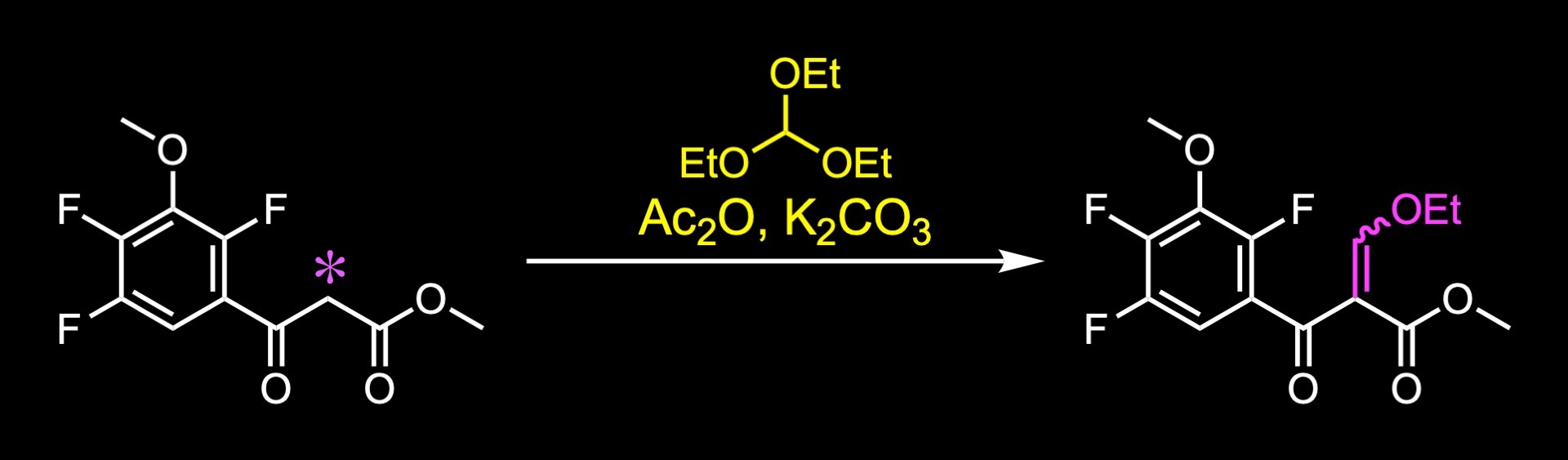
The fluoroquinolone core was constructed from this acetoacetate. The condensation with triethyl orthoformate in the presence of acetic anhydride and potassium carbonate gave the depicted product.
A plausible mechanism involves the formation of this active species by reaction between triethyl orthoformate and acetic anhydride. Then, the acidic proton of the keto ester can be abstracted by a mild base such as potassium carbonate, generating this enolate. The enolate can then attack this electrophile to generate this intermediate. Elimination of ethanol generates an ethoxymethylene product.
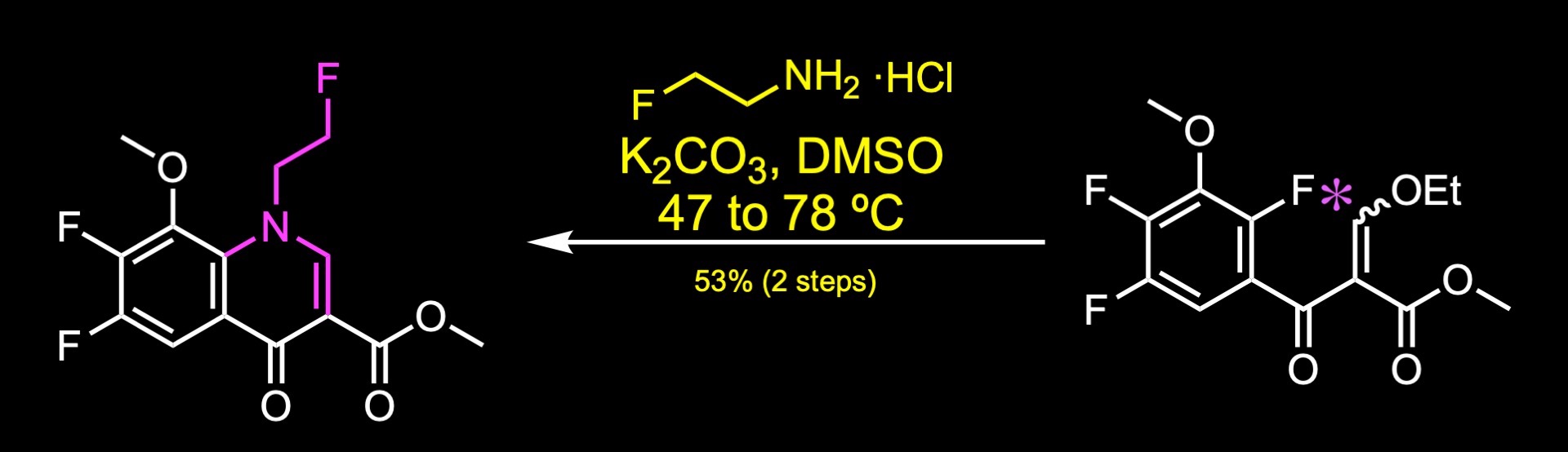
Treatment of this substrate with 2-fluoroethyl-amine hydrochloride generated the quinolone core of the molecule.
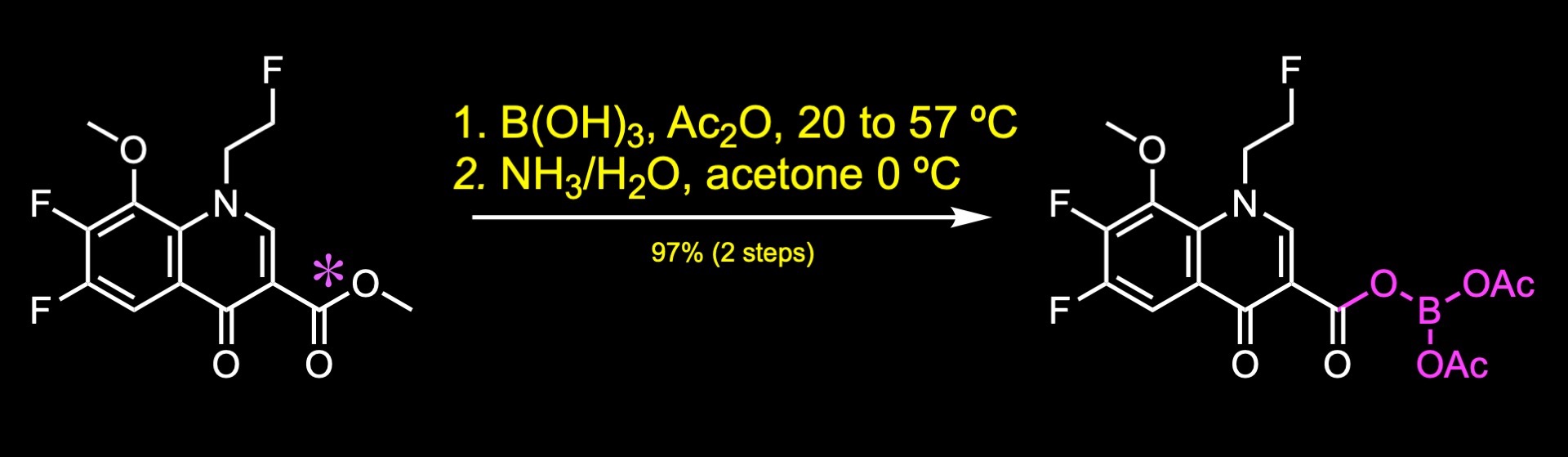
Then, the methyl ester was converted to a borate complex upon treatment with boric acid and acetic anhydride. According to the authors this transformation provided improved solubility and enhanced reactivity in the subsequent SNAr reaction.
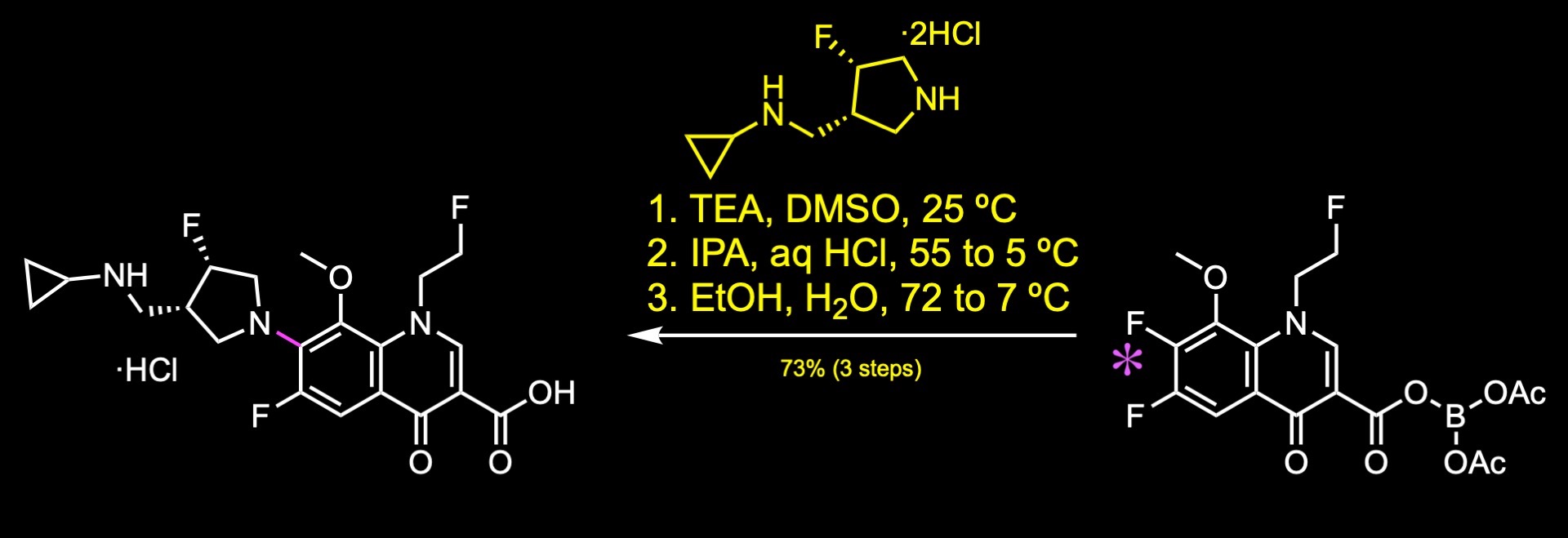
Finally, substitution of the aryl fluoride with the fluoropyrrolidine fragment provided lascufloxacin.
Reference work: