Futibatinib
Futibatinib Mechanism of Action
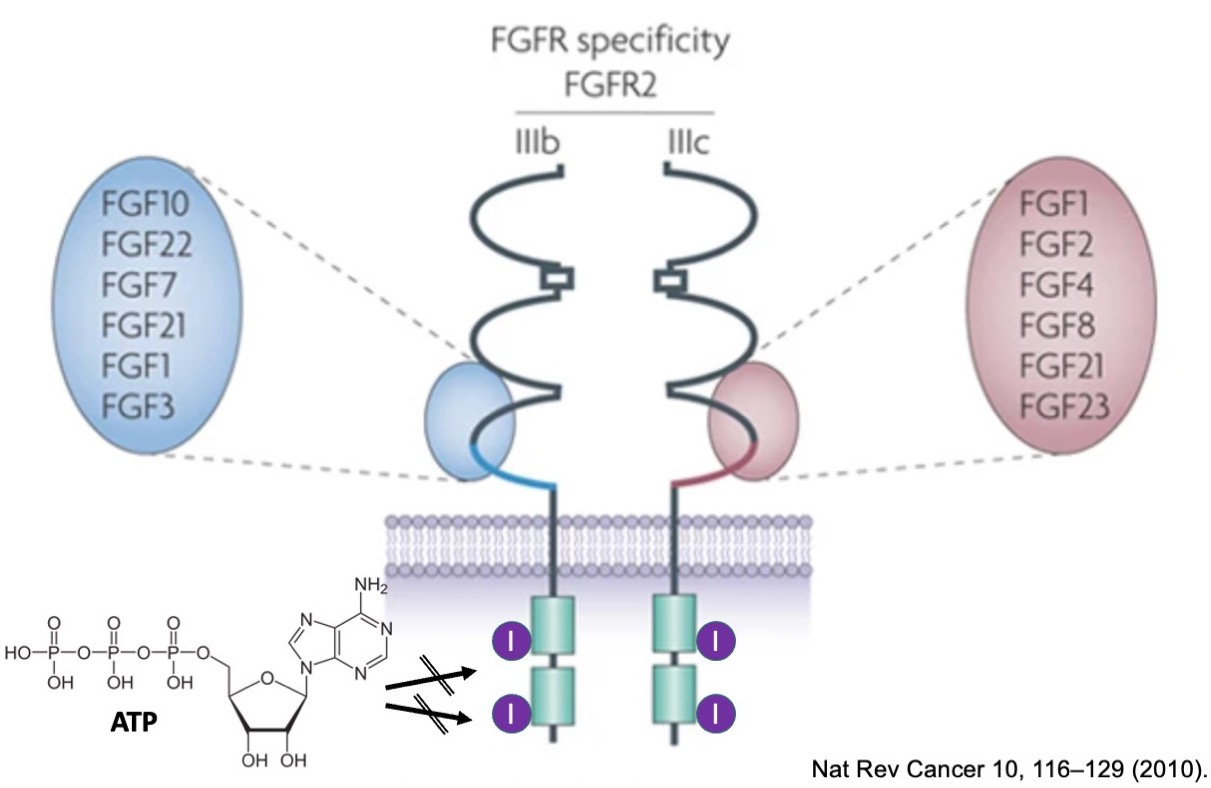
Fibroblast growth factor receptors (FGFR) are a family of receptors consisting of an extracellular region composed of a ligand-binding domain, a transmembrane segment, and a cytoplasmic tyrosine kinase domain. FGF receptor activation is triggered by the binding of one of the fibroblast growth factors to the extracellular receptor domain. Upon binding, the tyrosine kinase domains get phosphorylated by adenosine triphosphate, ATP, initiating a downstream cascade of intracellular signals. On a molecular level, these signals mediate cell division, growth, and differentiation. Alterations of FGF receptors have been identified to be important for progression and development of several types of cancer, including cholangiocarcinoma. In this setting, inhibition of FGF receptor signaling is a promising therapeutic strategy for cancer treatment. One modality of FGF receptor inhibitor is the use of small molecule compounds that bind to the ATP-binding site of the receptor at the tyrosine kinase domain and successfully block cross-phosphorylation and subsequent cell signaling.
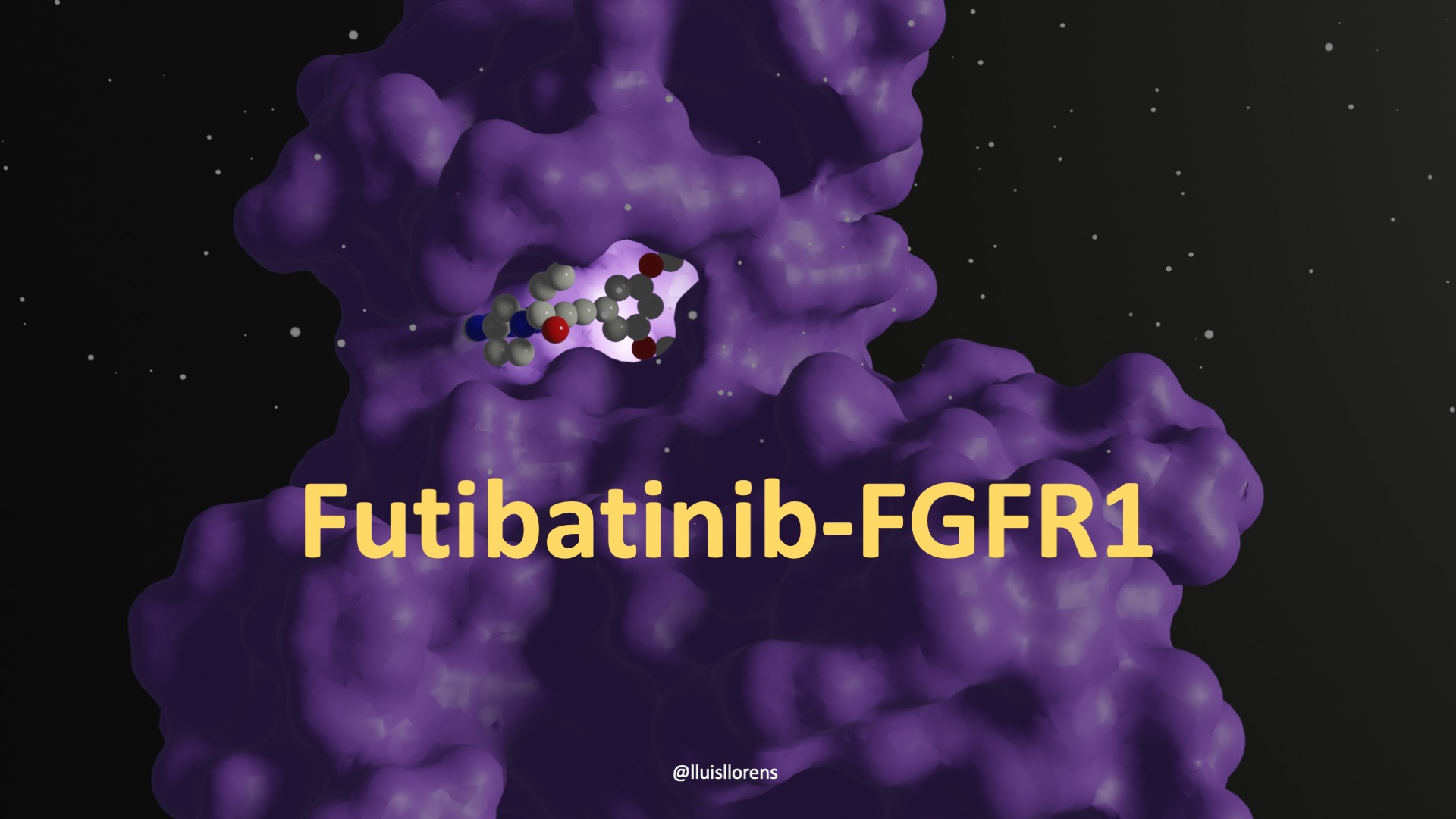
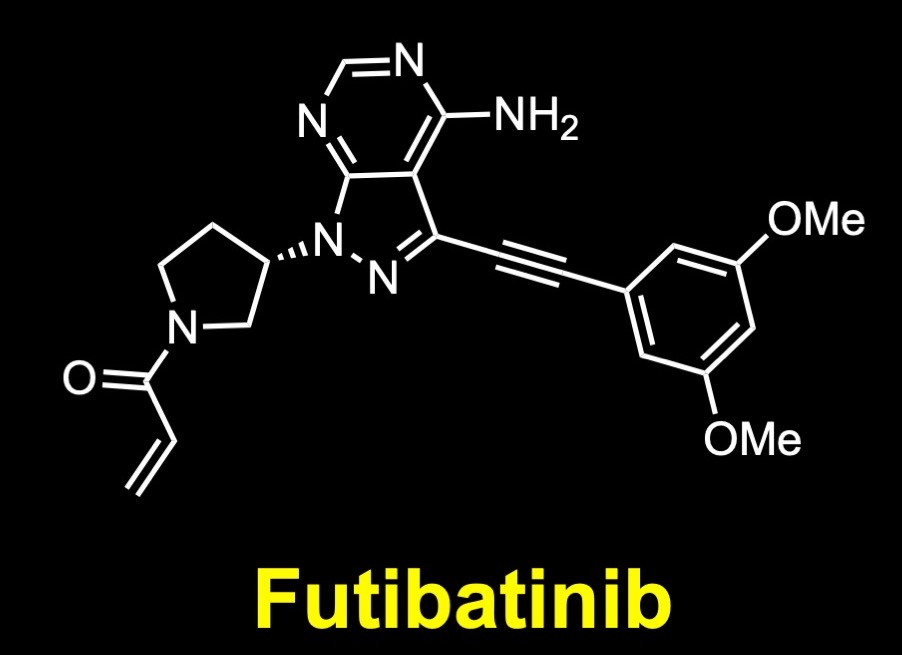
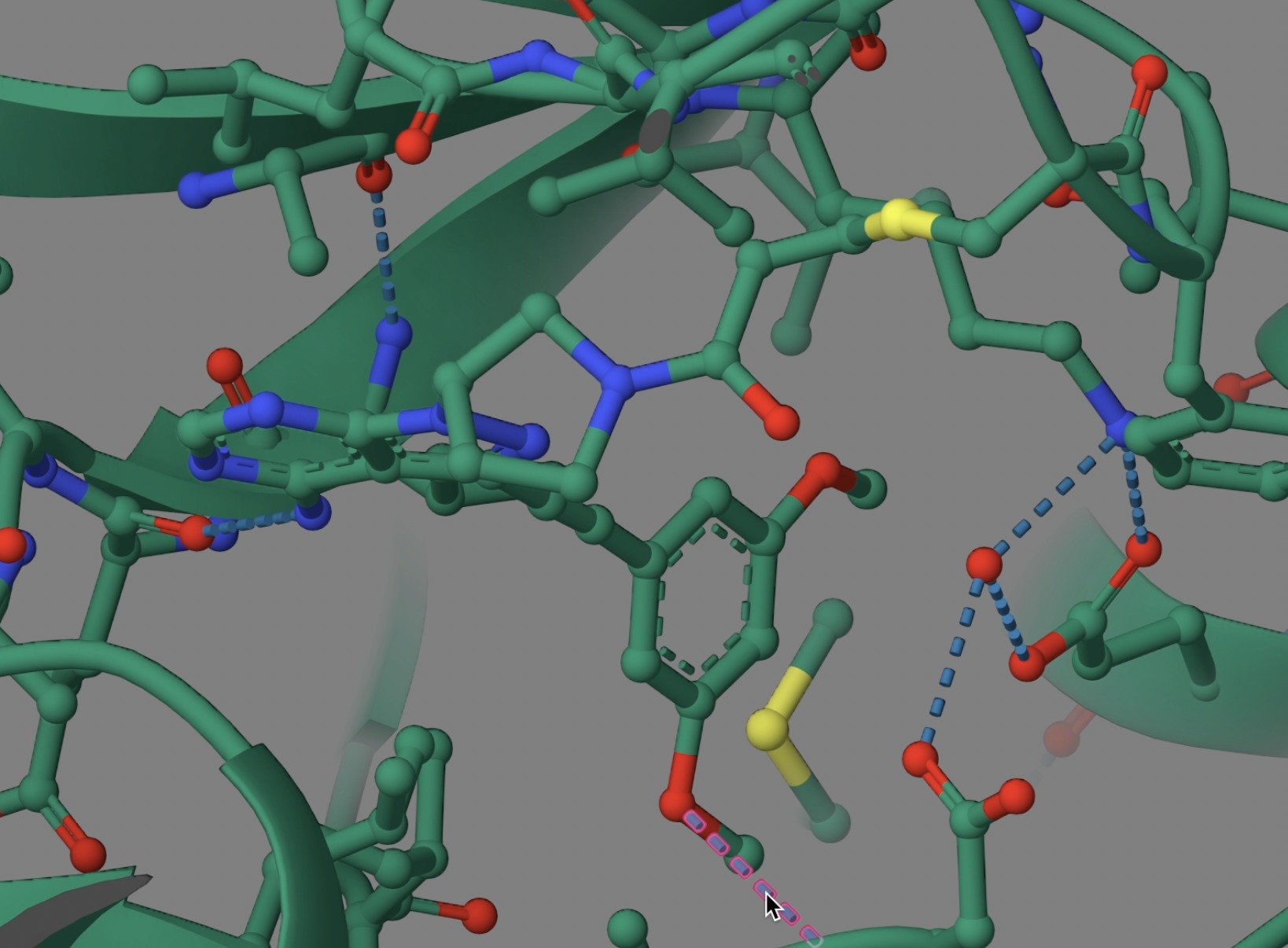
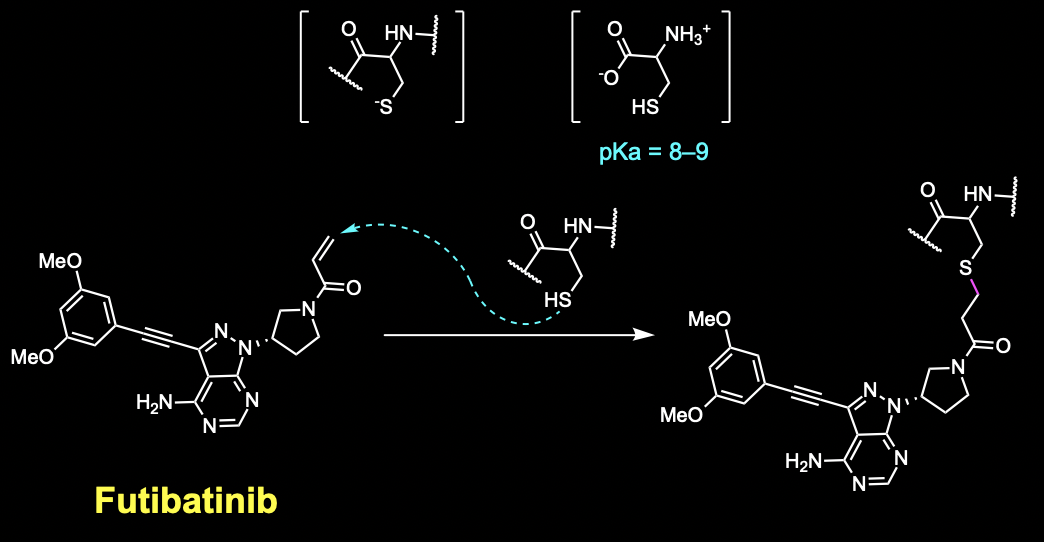
Binding at the ATP-binding site, it is no surprise that the molecule presents an ATP-mimicking pyrazolopyrimidine core structure in the middle, a pyrrolidine moiety overlapping with the sugar part of ATP, an acrylamide functional group at the edge, and a straight-chain alkyne to add the necessary chemical space for a dimethoxyphenyl ring to bind into a hydrophobic pocket of the receptor. The dimethoxyphenyl ring is stabilized by extensive van der Waals contacts and a single hydrogen bond (to Asp 641). The core pyrazolopyrimidine presents two hydrogen bonds (to Glu562 O and Ala564 N). And the acrylamide makes a covalent bond with the Cys488 S. (Futibatinib-FGFR 1 Covalent Complex: PDB ID: 6MZW.)
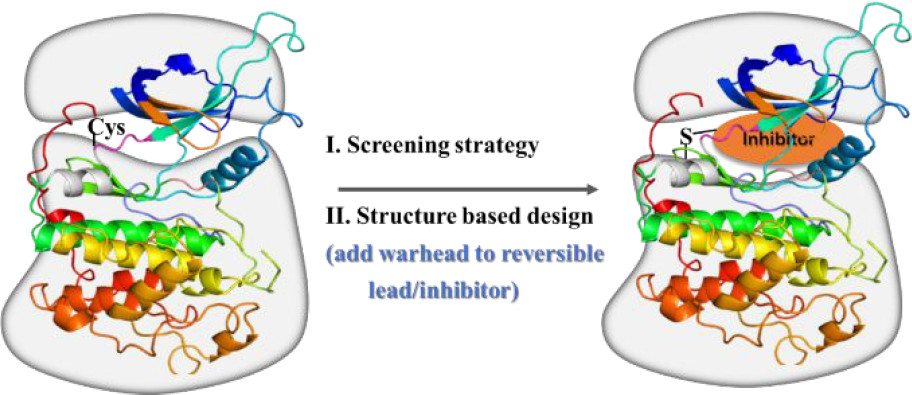
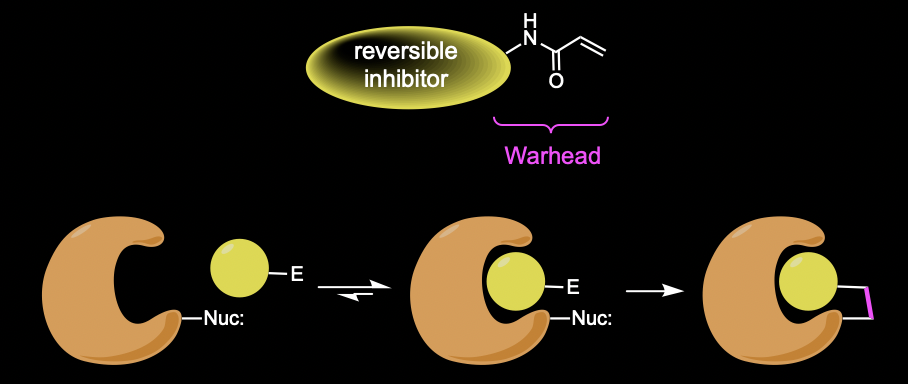
Futibatinib is a targeted covalent inhibitor (TCI). TCIs are typically generated by screening potential covalent binding compounds or by structure-based design from optimized reversible ligands, which are modified by attachment of an electrophilic covalent reactive group also known as warhead. Warheads are functional groups within a ligand that form covalent bonds with the targeted amino acid side chains in proteins. Futibatinib is a drug that was designed to undergo an irreversible hetero-Michael addition reaction with a unique cysteine residue of the specific protein.
TCI binding involves a two-step process in which an initial reversible binding event takes place, followed by the covalent bond-forming reaction. Irreversible inhibitors have the potential to be highly selective because they target a unique residue of a specific kinase by design, but warheads require a balanced reactivity profile. They should be reactive enough to form a covalent bond with the target residue, but only after the proper alignment of both reaction partners by the reversible binding event. Reactivity should be reduced to a necessary minimum in order to prevent nonspecific off-target labeling. In addition, warheads intended for in vivo use, either in drugs or in chemical probes, should also be chemically and metabolically stable and nontoxic.
Unfunctionalized acrylamides are weakly electrophilic and relatively unreactive toward thiols; this has led to acrylamides being the most successful electrophile used in targeted covalent inhibitors. On the other hand, the neutral cysteine thiol group is only moderately nucleophilic. However, this nucleophilicity is increased by several orders of magnitude for the thiolate form of the cysteine side chain, making it the strongest nucleophile among the 20 canonical amino acids. The thiol group can easily be deprotonated (pKa = 8–9 for cysteine), and the pKa can shift several orders of magnitude within proteins. Being highly polarizable, thiols and thiolates are soft bases. And the increased nucleophilicity and polarizability of thiolates compared to alkoxides are owed to the size and the high energy of the 3sp3 lone pairs.
Discovery of Futibatinib
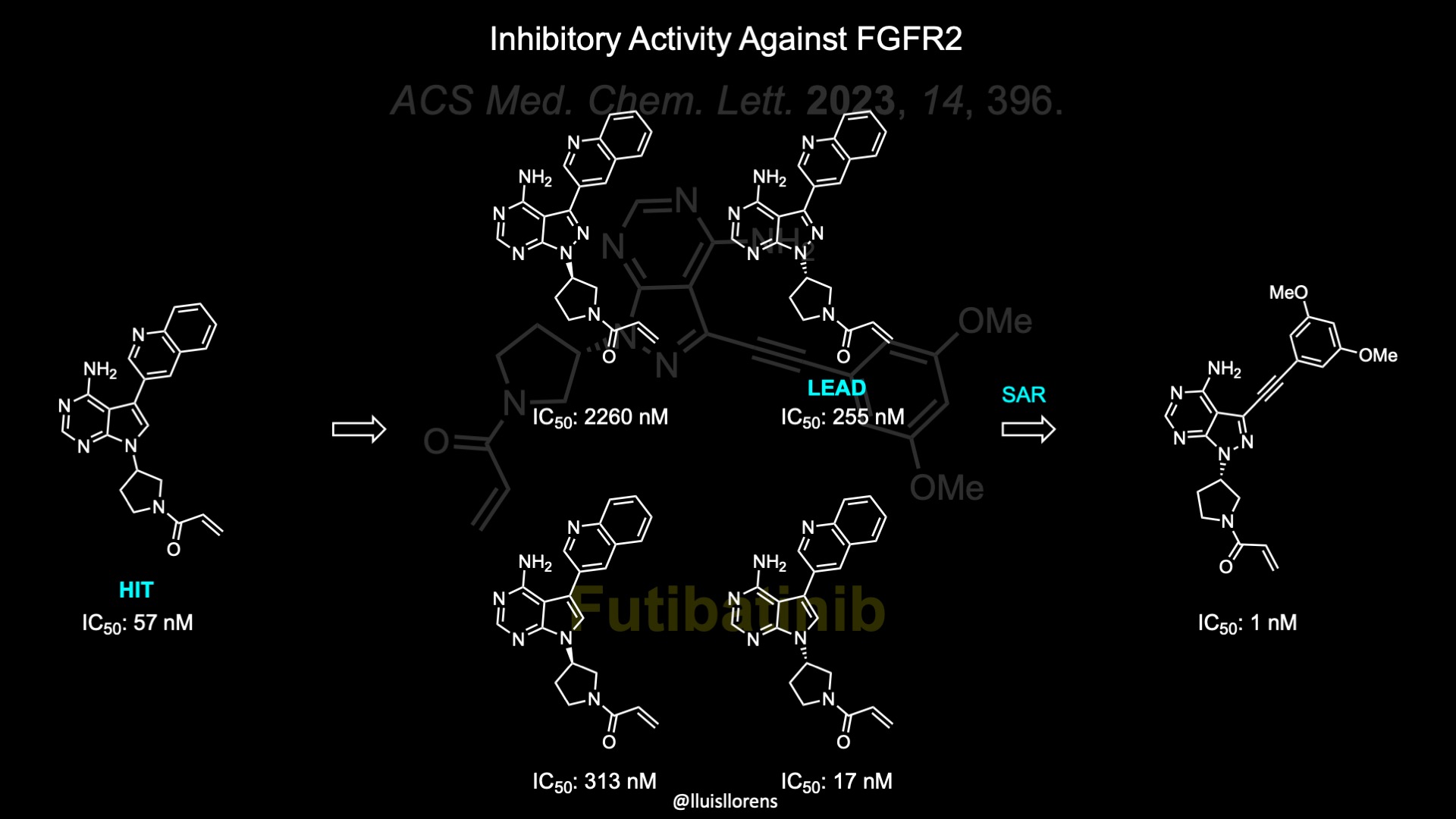
The discovery of futibatinib started off with the screening of an in-house compound collection where this pyrrolopyrimidine analog was identified as a unique hit with inhibitory activity of FGF receptor 2 with a half maximal inhibitory concentration (IC50) of 57 nM. The enantiomers of this compound and those of the pyrazolopyrimidine were prepared to investigate the effect of the stereochemistry of the pyrrolidine moiety and the additional nitrogen atom. The S-pyrrolidine analogs showed better inhibition. Although the pyrrolopyrimidine showed 15-fold more potent inhibitory activity than the pyrazolopyrimidine, the latter was selected as an initial lead molecule for rapid structure−activity relationship (SAR) generation, due to the synthetic accessibility of the pyrazolopyrimidine core. Considering that All FDA-approved FGFR-selective inhibitors possess a 3,5-dimethoxybenzene ring in their compounds, the Kinase inhibitory activity of 3,5-dimethoxybenzene analogs was tested. The straight-chain alkyne could correctly place the 3,5-dimethoxybenzene ring in the well-known unique hydrophobic pocket of the FGF receptor. Other linkers and warheads were also tested during this drug discovery campaign, which gave valuable information, but led to less potent compounds. For example, the propionamide analog, without covalent binding ability, was prepared to determine the effect of the acrylamide warhead moiety. This compound reduced the inhibitory activity by 240-fold, indirectly confirming the covalent inhibitory mechanism of futibatinib.
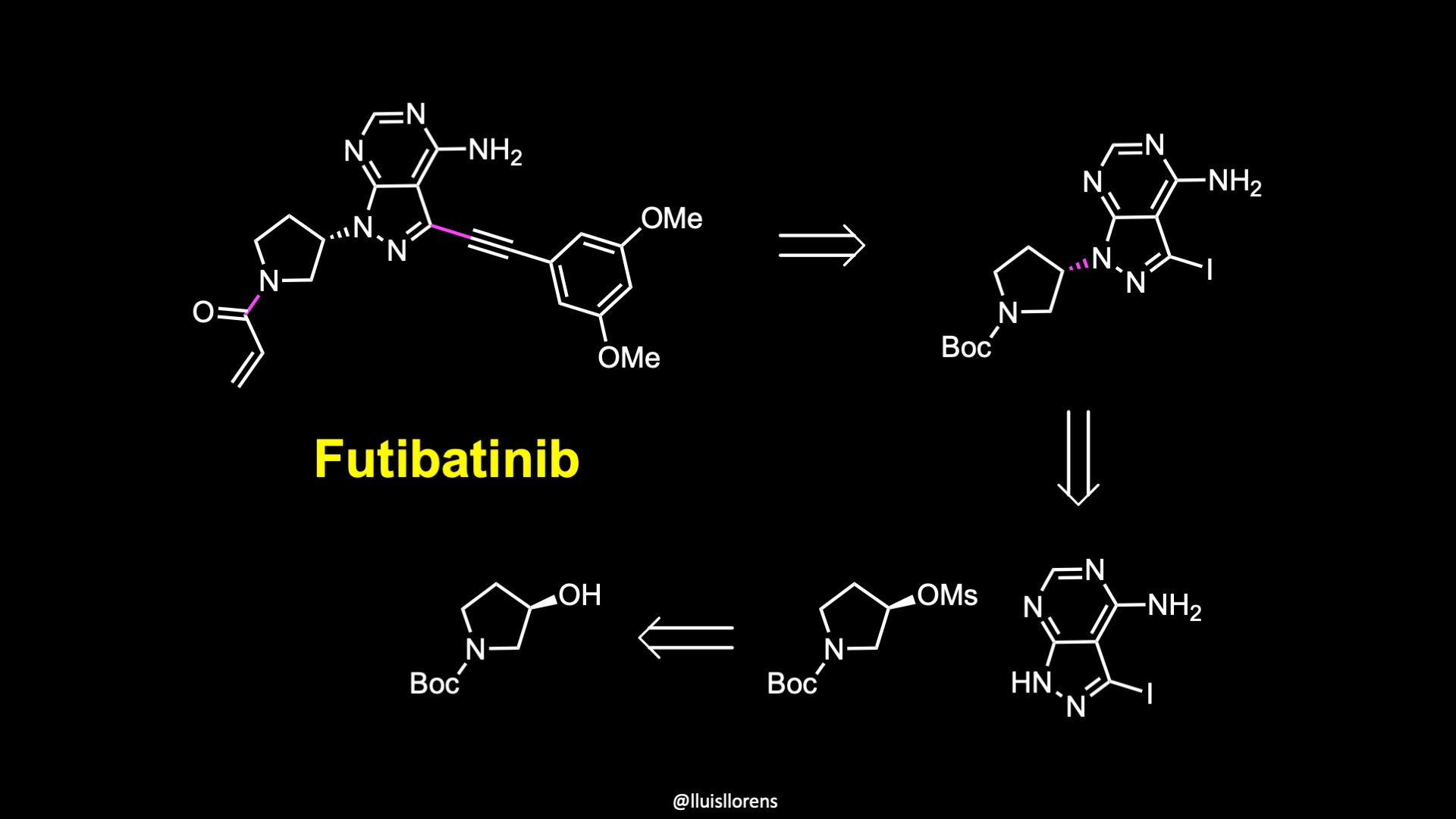
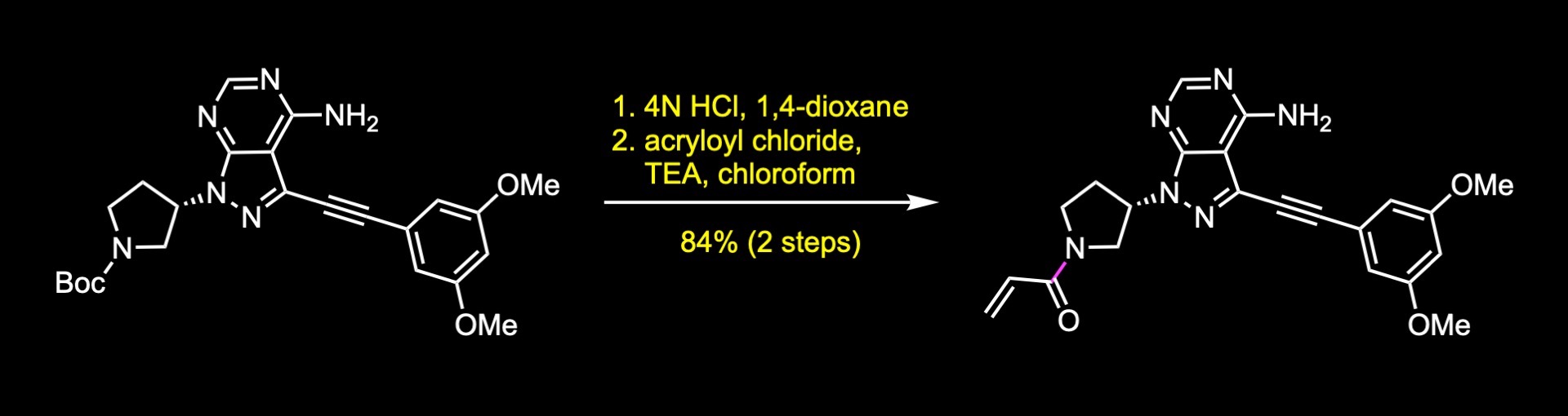
Synthesis of Futibatinib
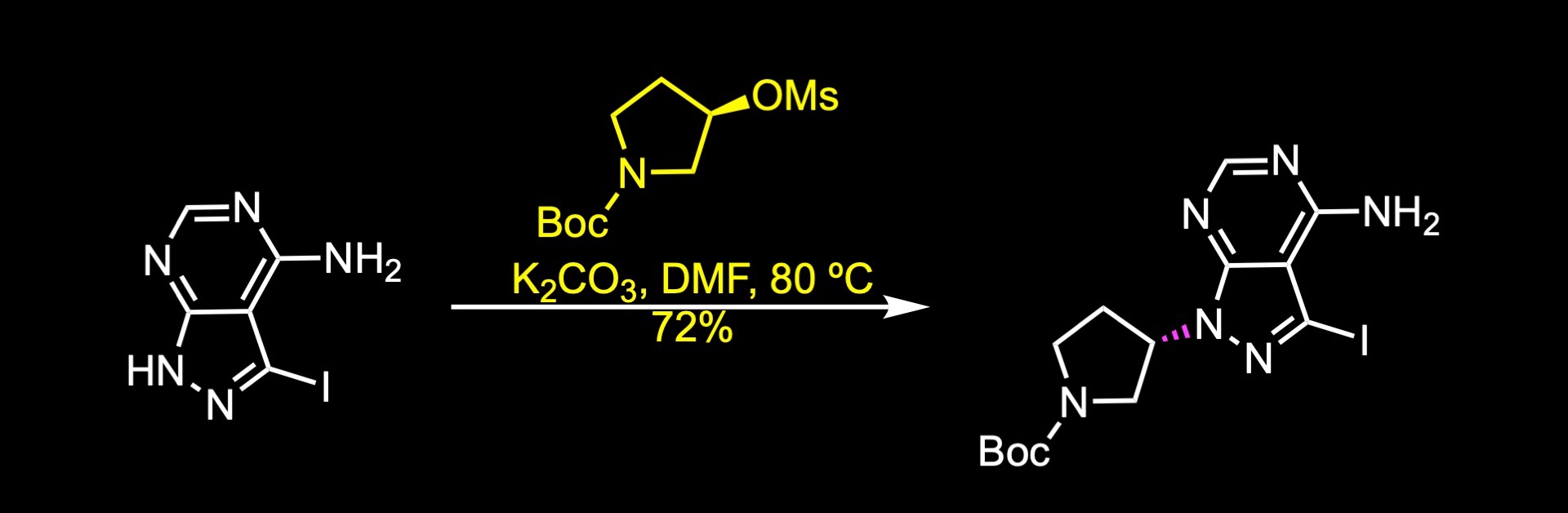
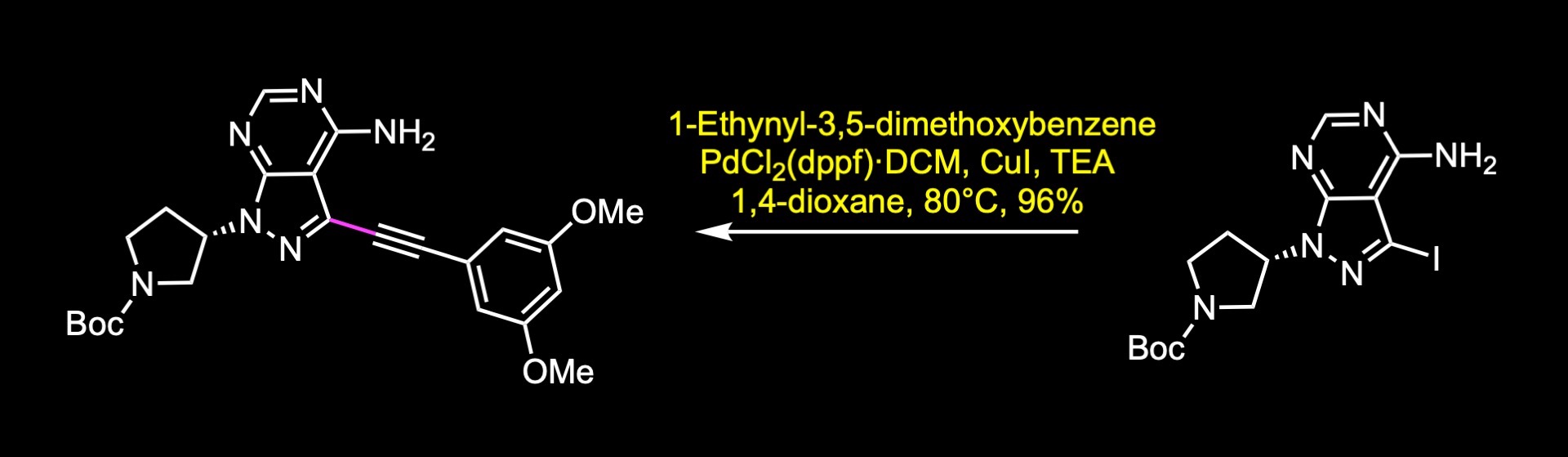
A short synthesis of the molecule was disclosed by researchers at Taiho pharmaceuticals. The key steps involved an acylation reaction to install the warhead, a carbon-carbon cross-coupling reaction, and an alkylation reaction. The first step of the synthesis was the nucleophilic substitution of the activated alcohol, which was generated in situ by the reaction of the corresponding alcohol with mesyl chloride. The next step was a Sonogashira reaction to install the internal alkyne. The reaction was accomplished in 96% yield under these conditions. Finally, boc cleavage and acylation reaction with acryloyl chloride delivered futibatinib in 84% yield over two steps.
References
Futibatinib:
1. ACS Med. Chem. Lett. 2023, 14, 4, 396–404.
2. ChemMedChem 2019, 14, 494.
FGFR:
1. Nat Rev Cancer 10, 116–129 (2010).
Covalent Inhibitors and Warheads:
1. J. Med. Chem. 2022, 65, 1, 58–83.
2. Current Opinion in Chemical Biology 2017, 39:54–63.
3. J. Med. Chem. 2019, 62, 12, 5673–5724.
Futibatinib-FGFR 1 Covalent Complex: PDB ID: 6MZW.