Synthesis of Bempedoic Acid
Biology Notes
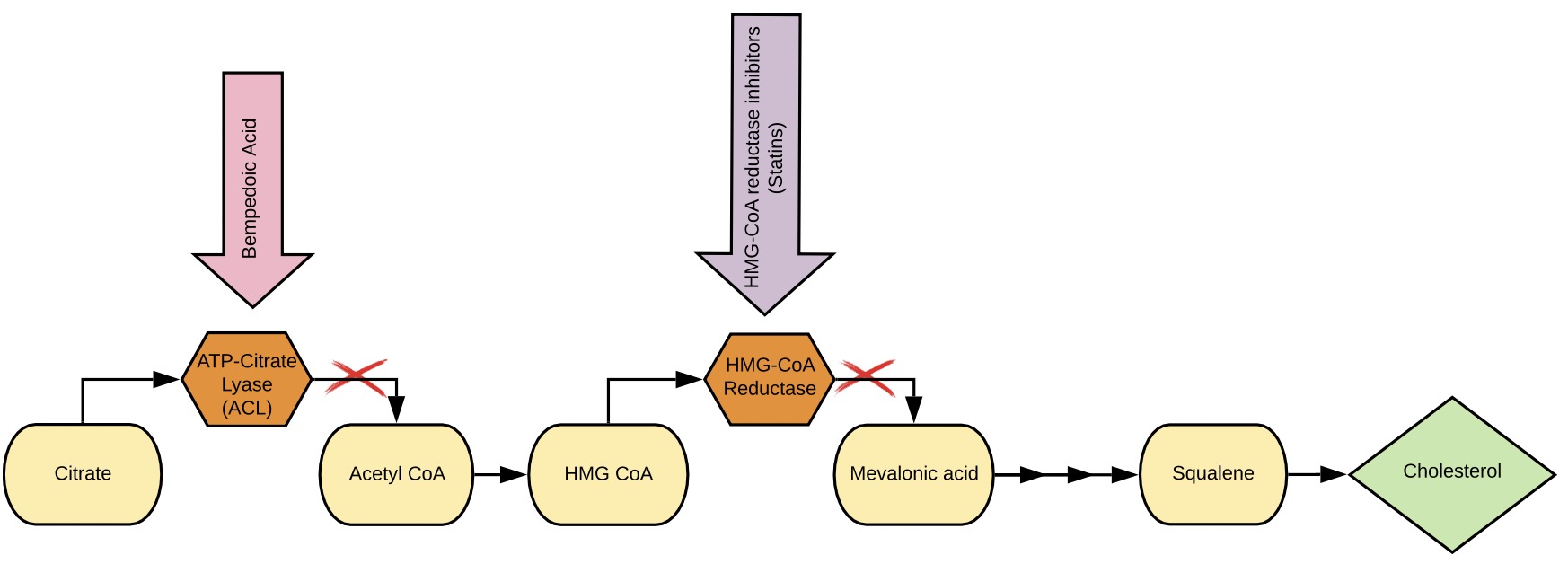
Cholesterol is an essential structural component of cell membranes in the human body and serves as a precursor for the biosynthesis of numerous compounds such as steroid hormones, cell membranes, and vitamin D. Cholesterol can be obtained through diet or endogenously synthesized within hepatocytes. Disruption in cholesterol homeostasis due to hereditary factors or diet can lead to elevated plasma cholesterol levels that can produce fatty deposits in blood vessels, which, over time, can impede proper blood flow.
Bempedoic acid is a medicine that lowers low-density lipoprotein cholesterol (LDL-c; also known as ‘bad’ cholesterol). It does so by blocking adenosine triphosphate citrate lyase, which is an enzyme in the liver that is responsible for making cholesterol. As a result, bempedoic acid lowers blood levels of cholesterol, including LDL-cholesterol and other fatty substances made by the liver. Bempedoic acid is a prodrug. A prodrug is a pharmacologically inactive medication that, after intake, is metabolized into a pharmacologically active drug. It is activated to the thioester with coenzyme A in the liver. According to the mechanism of action, the active form of bempedoic acid reduces the production of cytosolic acetyl-coenzyme A (acetyl-CoA), the final substrate for both fatty acid and sterol synthesis. [2]
Retrosynthetic Analysis
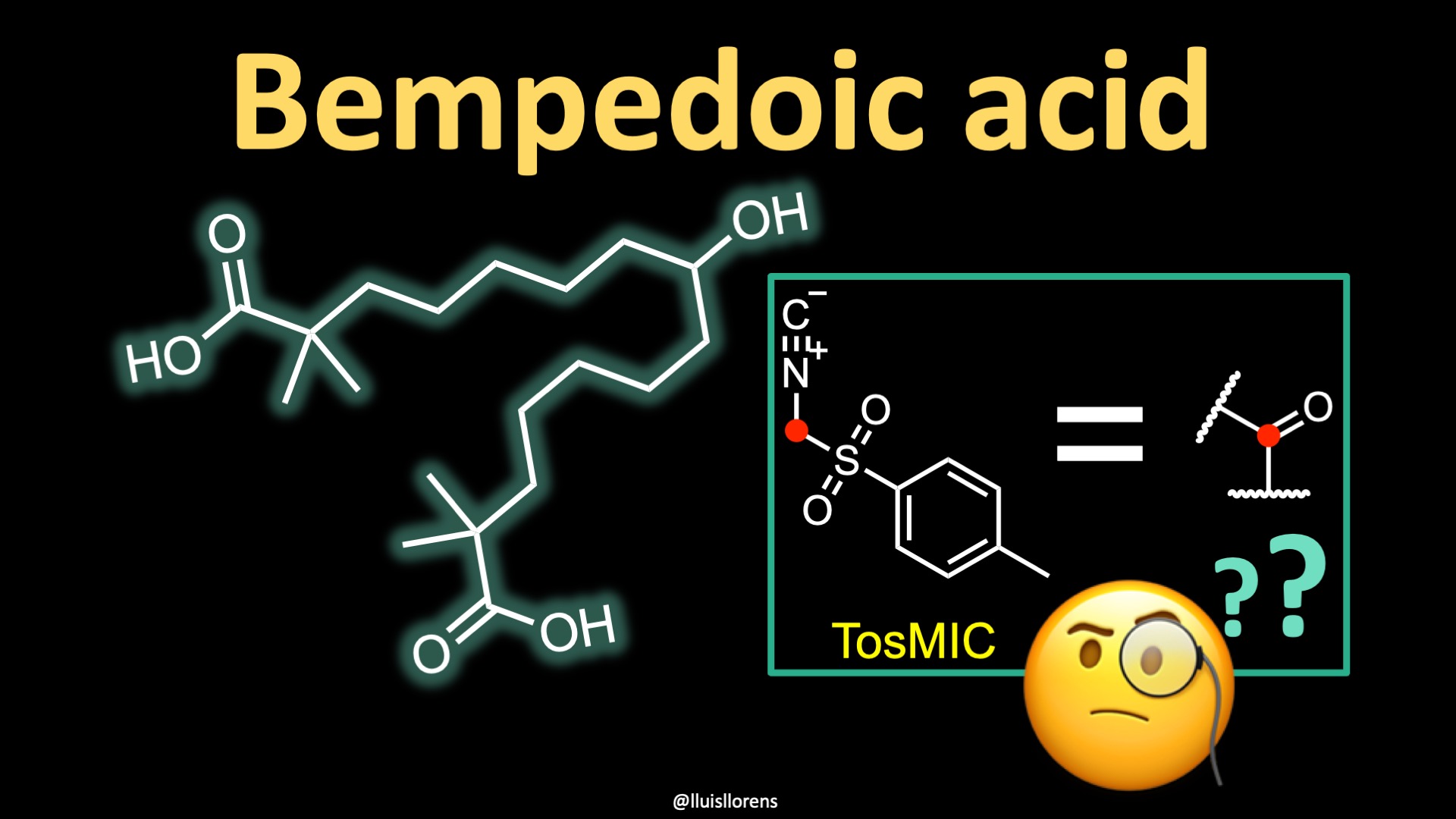
The chemical structure of the molecule resembles that of oleic acid. It has been classified in the literature as a fraudulent fatty acid, because it belongs to a family of molecules that activate regulatory pathways in the liver, resulting in enhanced fatty acid catabolism. [3] These molecules are characterized by elevated hydrophobicity and the frequent presence of carboxylic acid functional groups. In fact, bempedoic acid presents two carboxylic acids, two spacer groups or symmetrical alkyl chains, and a central group.
A convenient route for the synthesis of this molecule would involve breaking the two C–C bonds adjacent to the hydroxyl group, because that would deliver two identical fragments. However, both the carbonyl group and the alkyl halide are electrophiles. Therefore, umpolung of one of the functional groups is required. Researchers at Esperion Therapeutics made use of a ketone surrogate that was employed as the nucleophilic counterpart.
Synthesis of Bempedoic Acid
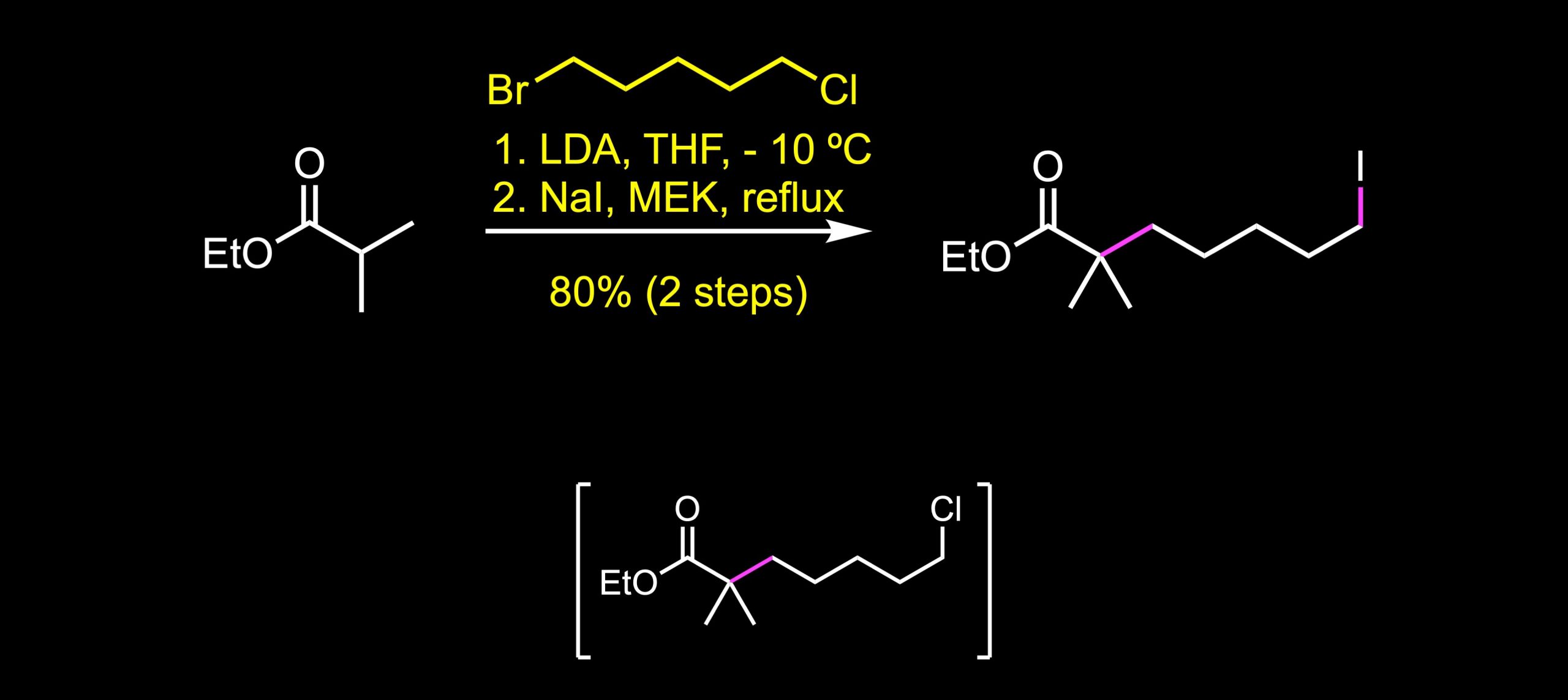
The synthesis begins with the alkylation reaction with the alkyl bromide. LDA generates the enolate that displaces the bromine atom to deliver the intermediate product that is subjected to a Finkelstein reaction to afford the alkyl iodide in 80% yield over two steps. The Finkelstein reaction is an SN2 reaction that involves the exchange of one halogen atom for another. It is an equilibrium reaction, but the reaction can be driven to completion by exploiting the differential solubility of halide salts, or by using a large excess of the halide salt. While sodium iodide is soluble in methyl ethyl ketone, sodium chloride is not.
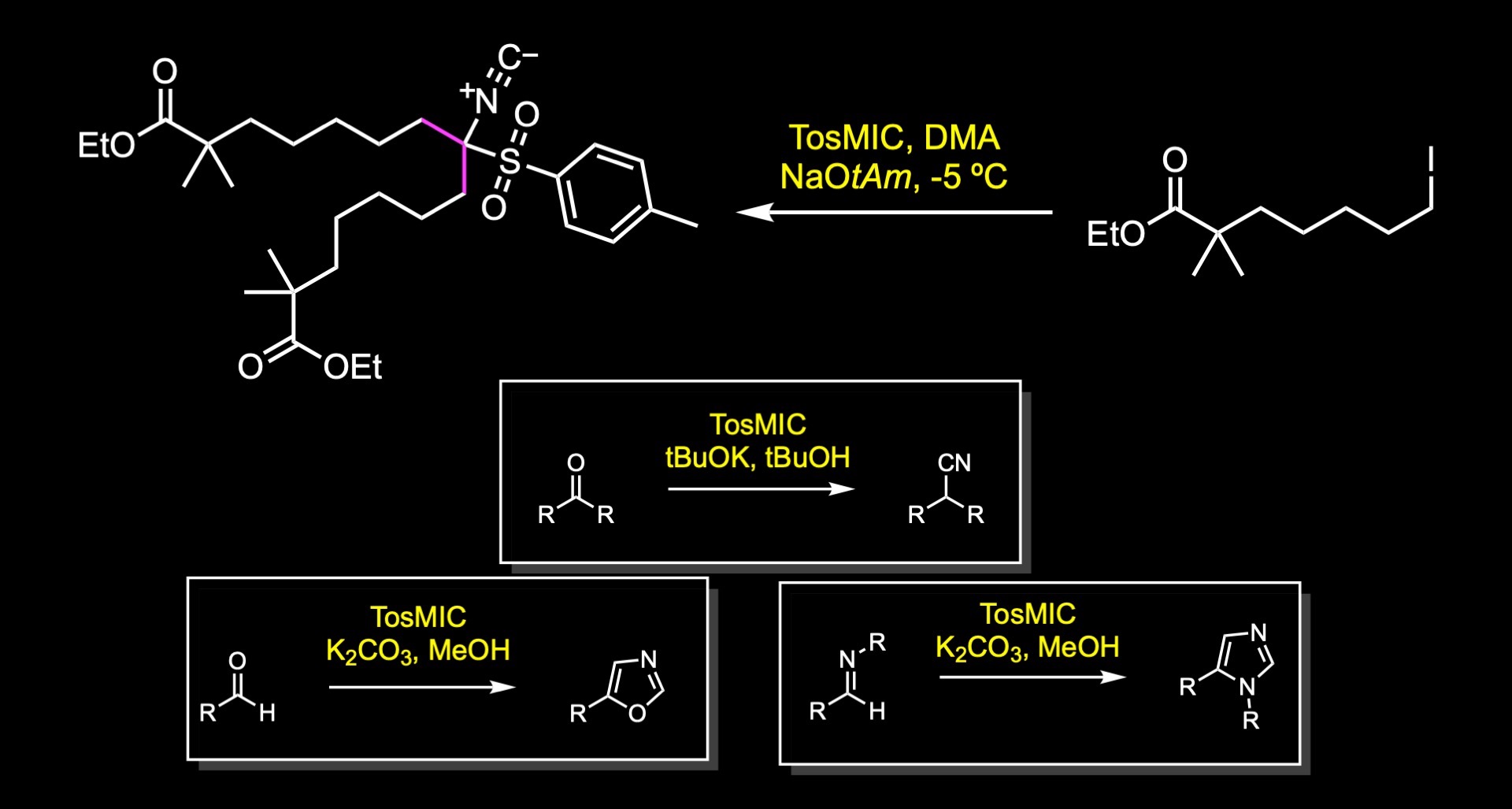
This material is then reacted with 0.5 equivalents of toluenesulfonylmethyl isocyande under basic conditions to facilitate a double alkylation reaction of the iodide to obtain the isonitrile.
TosMIC is a commercially available colorless solid. It contains both sulfonyl and isocyanide groups. The isocyano group acts as an electron acceptor of strength comparable to an ester group, making the alpha carbon acidic by nature. The reagent is often used in the Van Leusen reaction to convert ketones to nitriles or in the preparation of oxazoles and imidazoles.
The isonitrile is then subjected to acidic conditions in isopropyl acetate and pH adjustment to uncover the masked carbonyl group. These two last steps highlight the versatility of TosMIC reagent as a ketone surrogate where the carbon of the resulting carbonyl group is employed as a nucleophile.
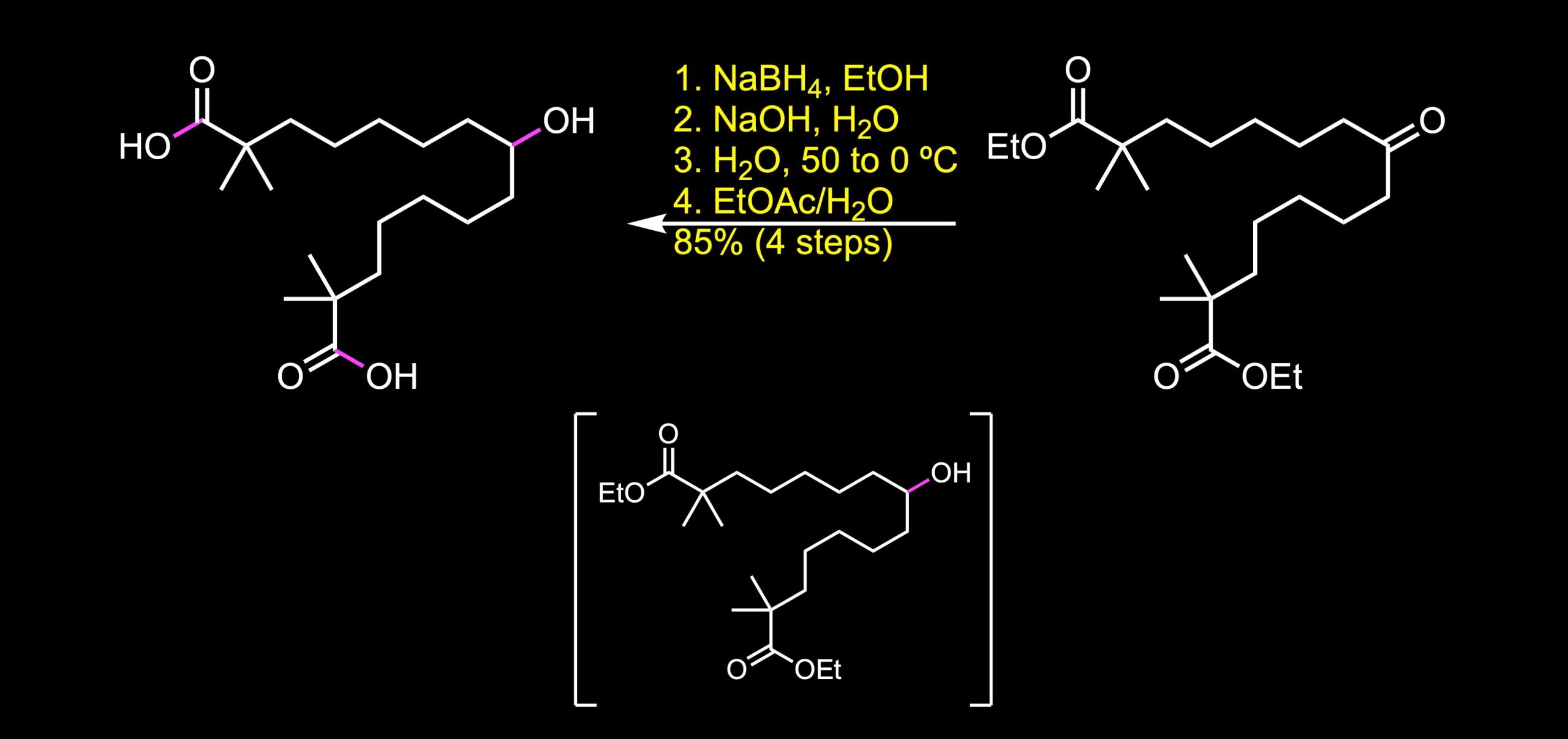
Reduction of the ketone with sodium borohydride followed by saponification and further purification and crystallization steps give rise to bempedoic acid in an excellent overall yield.