Sonogashira Coupling
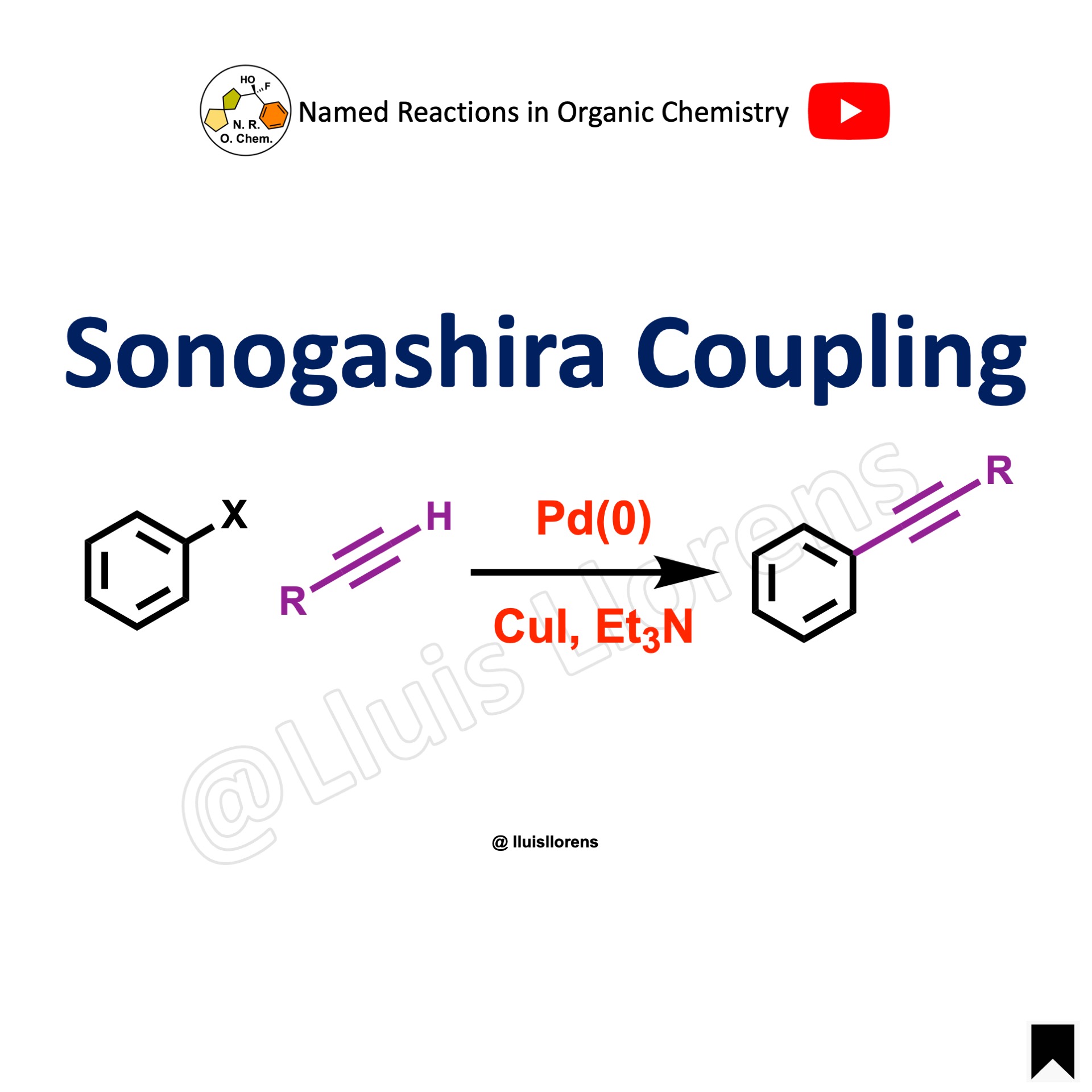
The Sonogashira cross-coupling is the copper-palladium catalyzed reaction of terminal alkynes with aryl or vinyl halides.
General features:
1. It can be carried out at room temperature. 2. The base can serve as the solvent but a co-solvent can be used. 3. A wide range of functional groups is tolerated on the aromatic or vinyl halide substrates. 4. Some aryl halides and bulky substrates may require higher temperatures, which can lead to side reactions. 5. The reactivity of the aryl and vinyl halides is (from most to least reactive) I, OTf, Br, Cl. Selective coupling with the iodides in the presence of bromides.
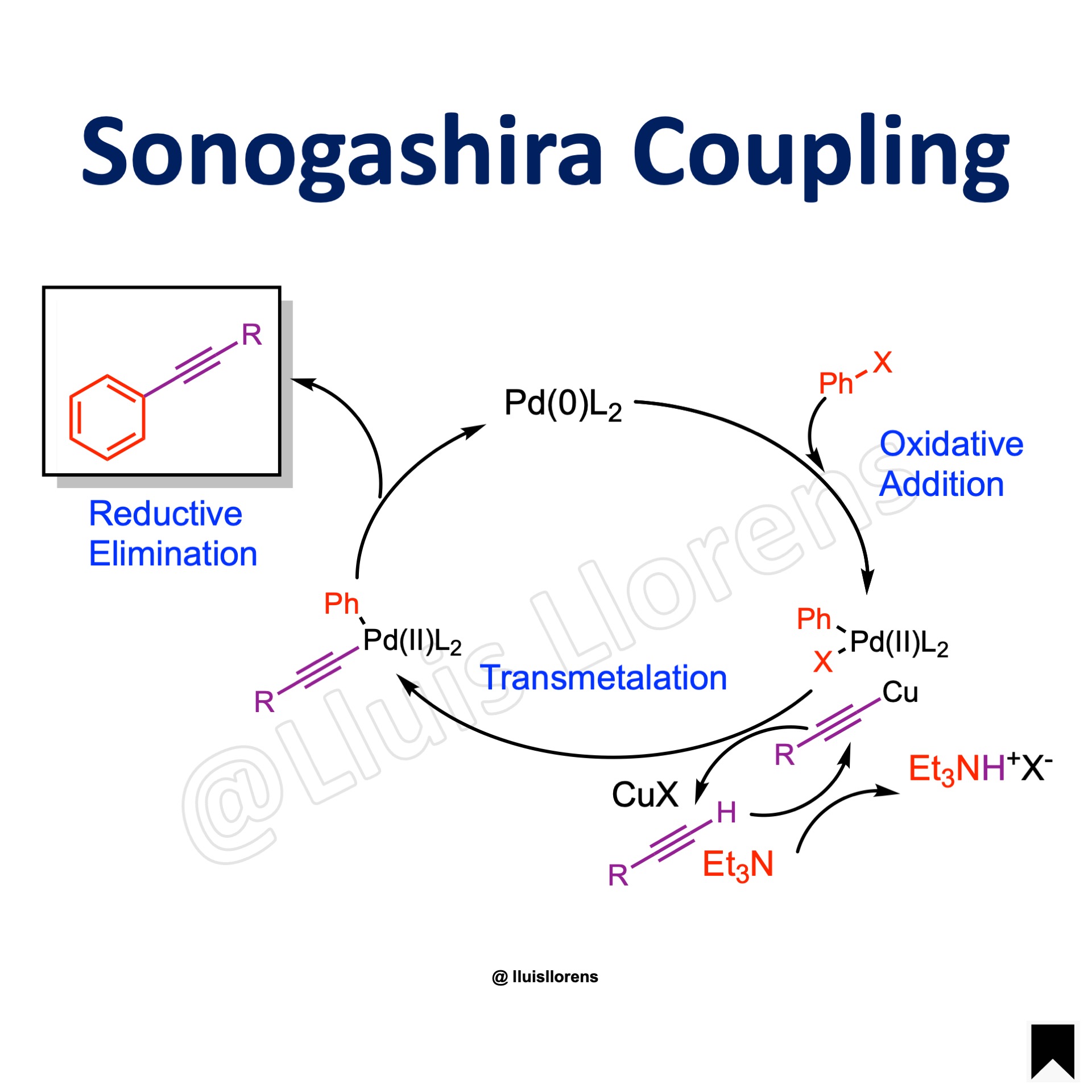
Reaction Mechanism
1. Oxidative addition of an organic halide to Pd(0) to form Pd(II). 2. Deprotonation of the alkyne and formation of copper acetylide. 3. Transmetalation between Pd(II) and the acetylide. 4. Reductive elimination to form the C–C sigma bond and regeneration of the catalyst.
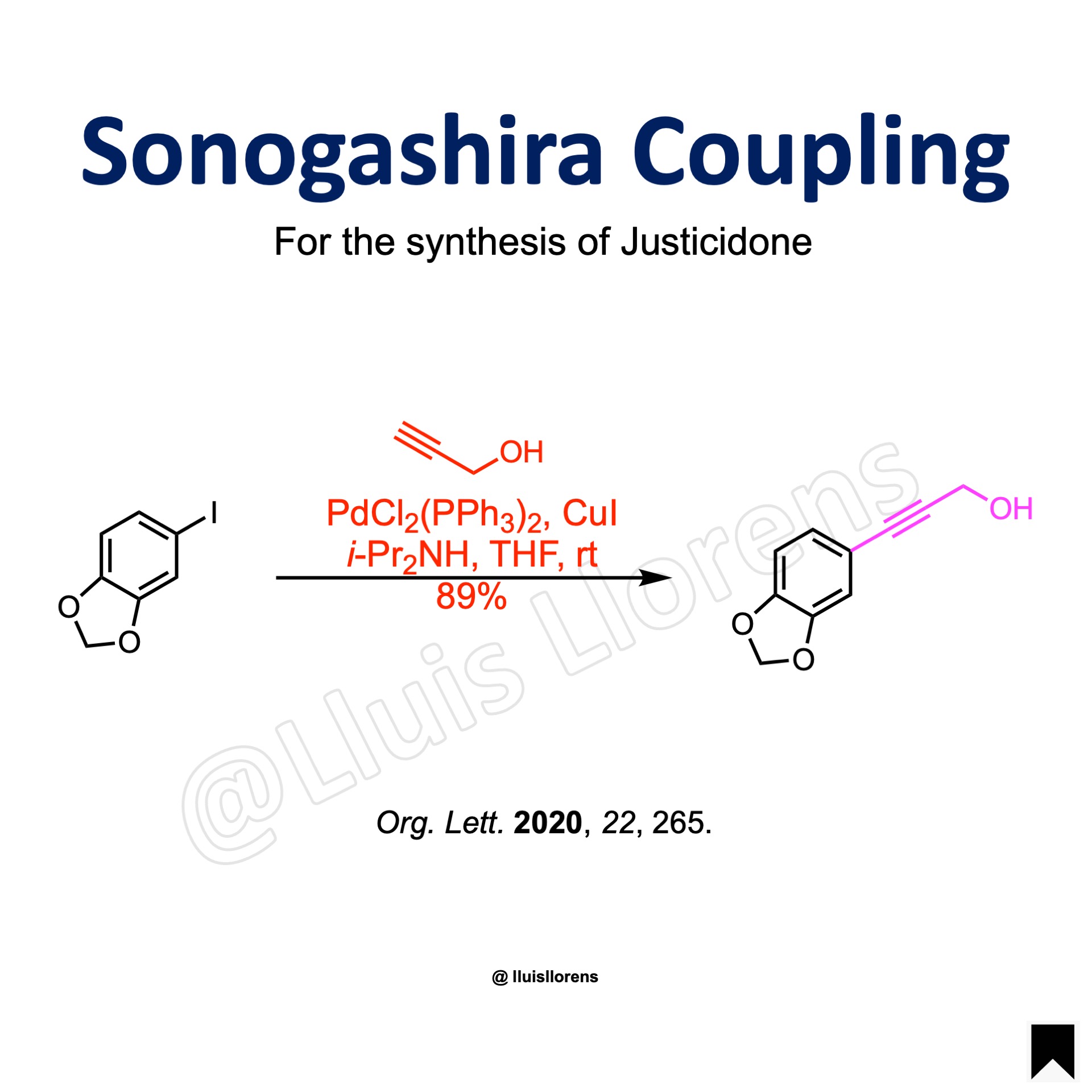
Example
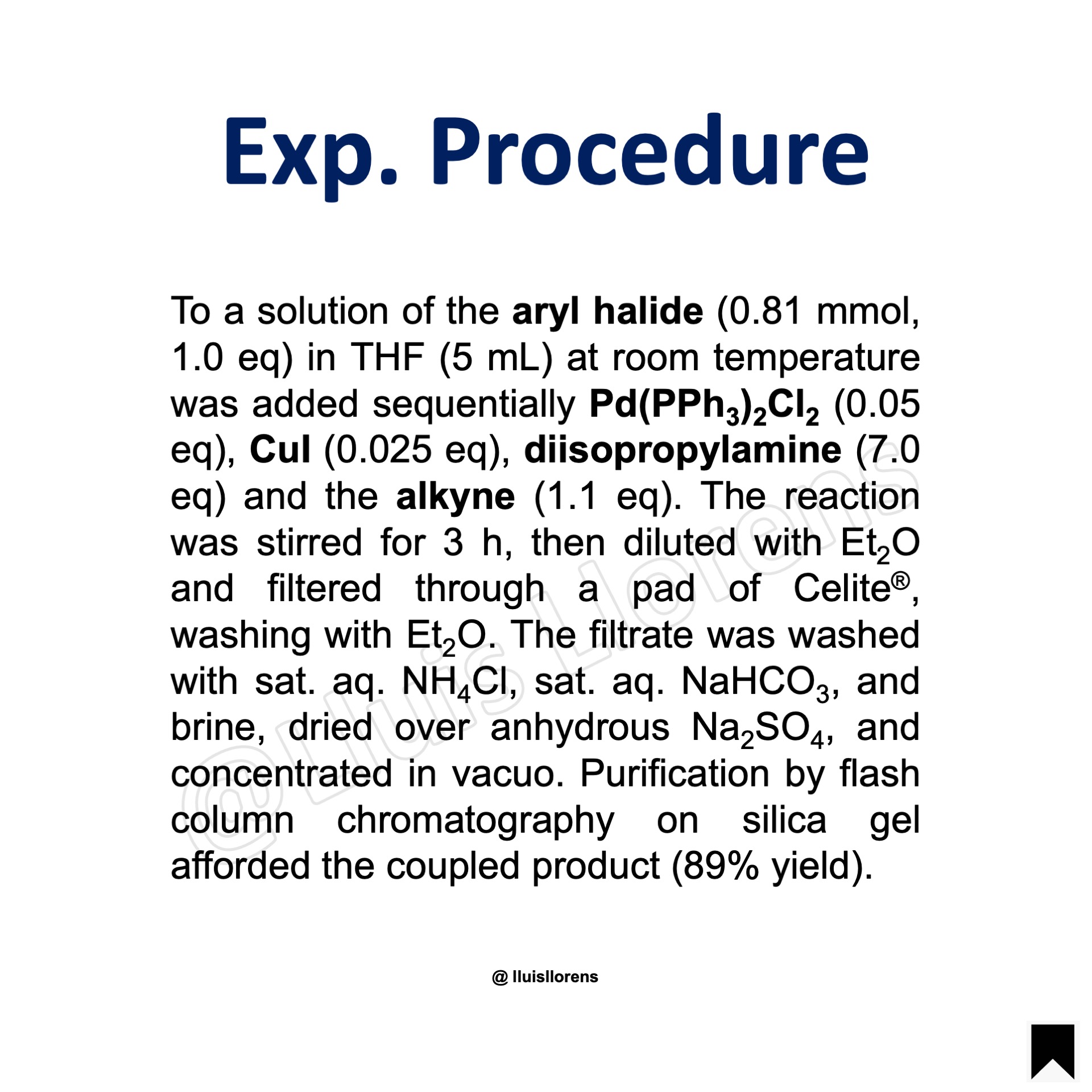
Experimental Procedure
To a solution of the aryl halide (0.81 mmol, 1.0 eq) in THF (5 mL) at room temperature was added sequentially Pd(PPh3)2Cl2 (0.05 eq), CuI (0.025 eq), diisopropylamine (7.0 eq) and the alkyne (1.1 eq). The reaction was stirred for 3 h, then diluted with Et2O and filtered through a pad of Celite®, washing with Et2O. The filtrate was washed with sat. aq. NH4Cl, sat. aq. NaHCO3, and brine, dried over anhydrous Na2SO4, and concentrated in vacuo. Purification by flash column chromatography on silica gel afforded the coupled product (89% yield).
Learn More Named Reactions
[instagram-feed feed=2]