Simmons-Smith Reaction
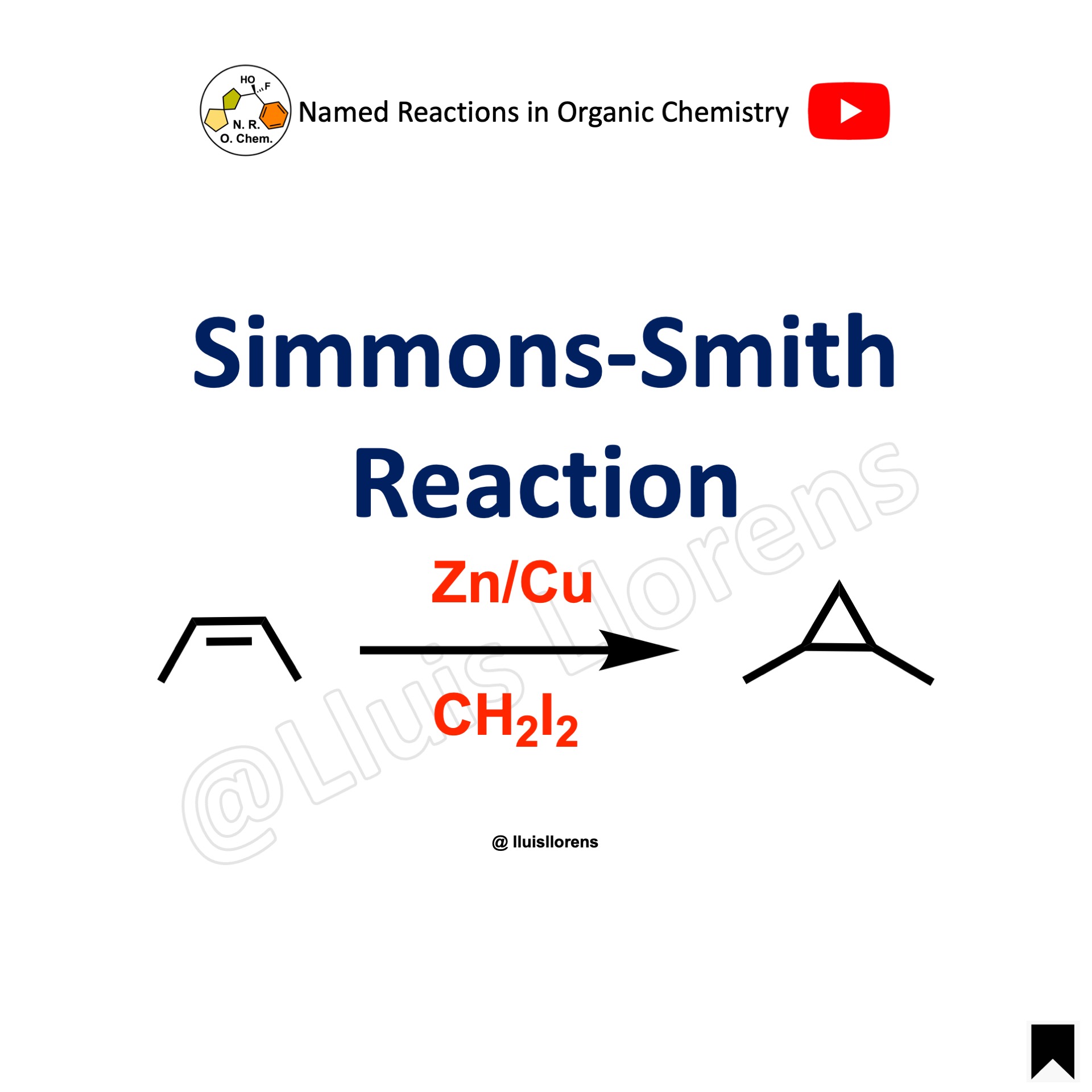
The Simmons-Smith reaction is a ring-forming reaction that allows the transformation of cyclopropanes by the reaction of alkenes with diiodomethane in the presence of zinc–copper couple.
General features:
1. A wide range of alkenes can be used: simple olefins, α,β-unsaturates ketones and aldehydes, electron-rich alkenes such as enol ethers, or enamines… 2. The cyclopropanation is stereospecific; the stereochemical information in the alkene is translated to the product. 3. Side reactions are seldom observed, and the reaction conditions are tolerant of most functional groups. 4. non-Coordinating solvents such as DCM or DCE are recommended because the use of basic solvents decreases the rate of the reaction.
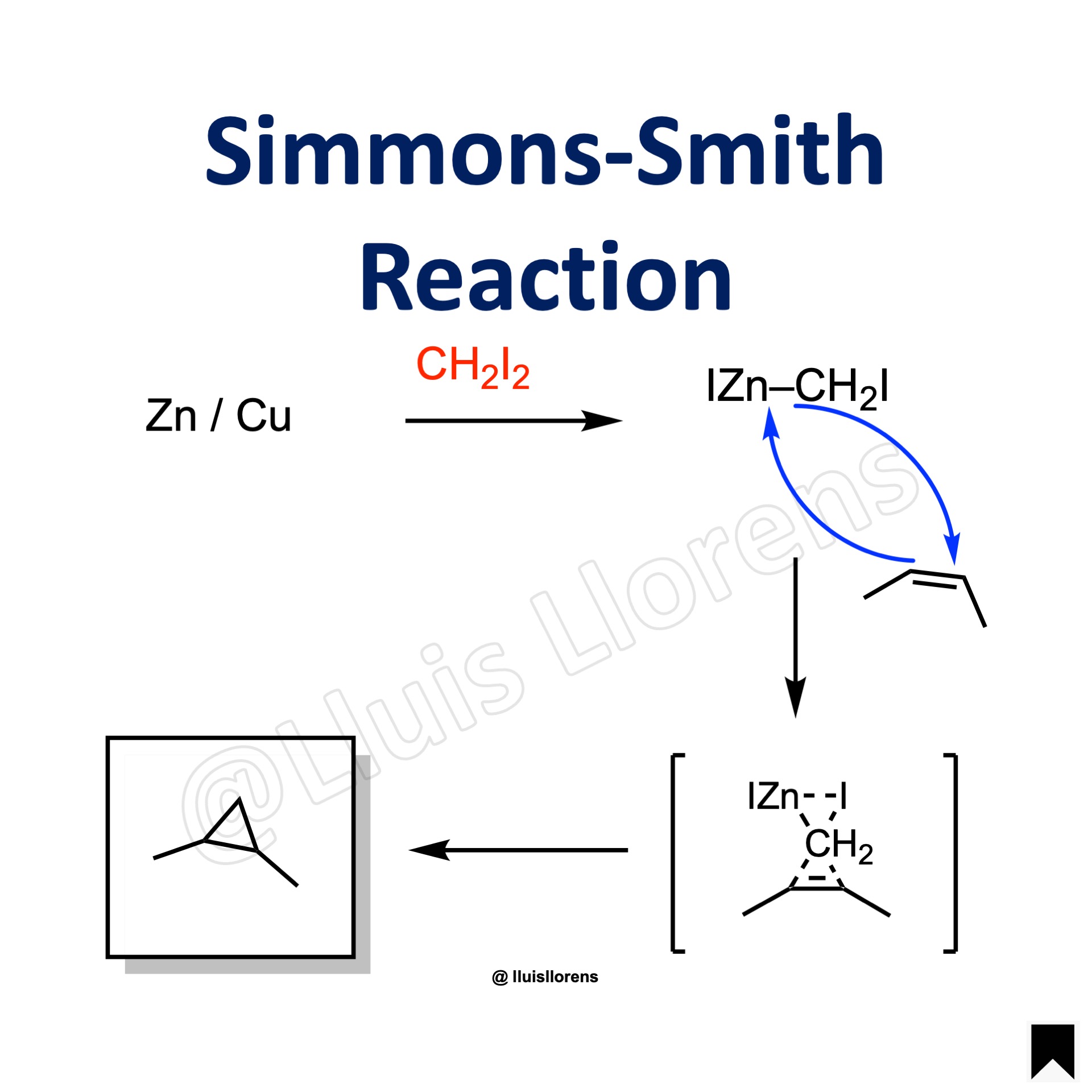
Reaction Mechanism
1. Formation of the organozinc carbenoid. 2. Reaction with the alkene delivers the corresponding cyclopropane via a concerted process through a three-centered “butterfly-type” transition state.
Experimental Procedure
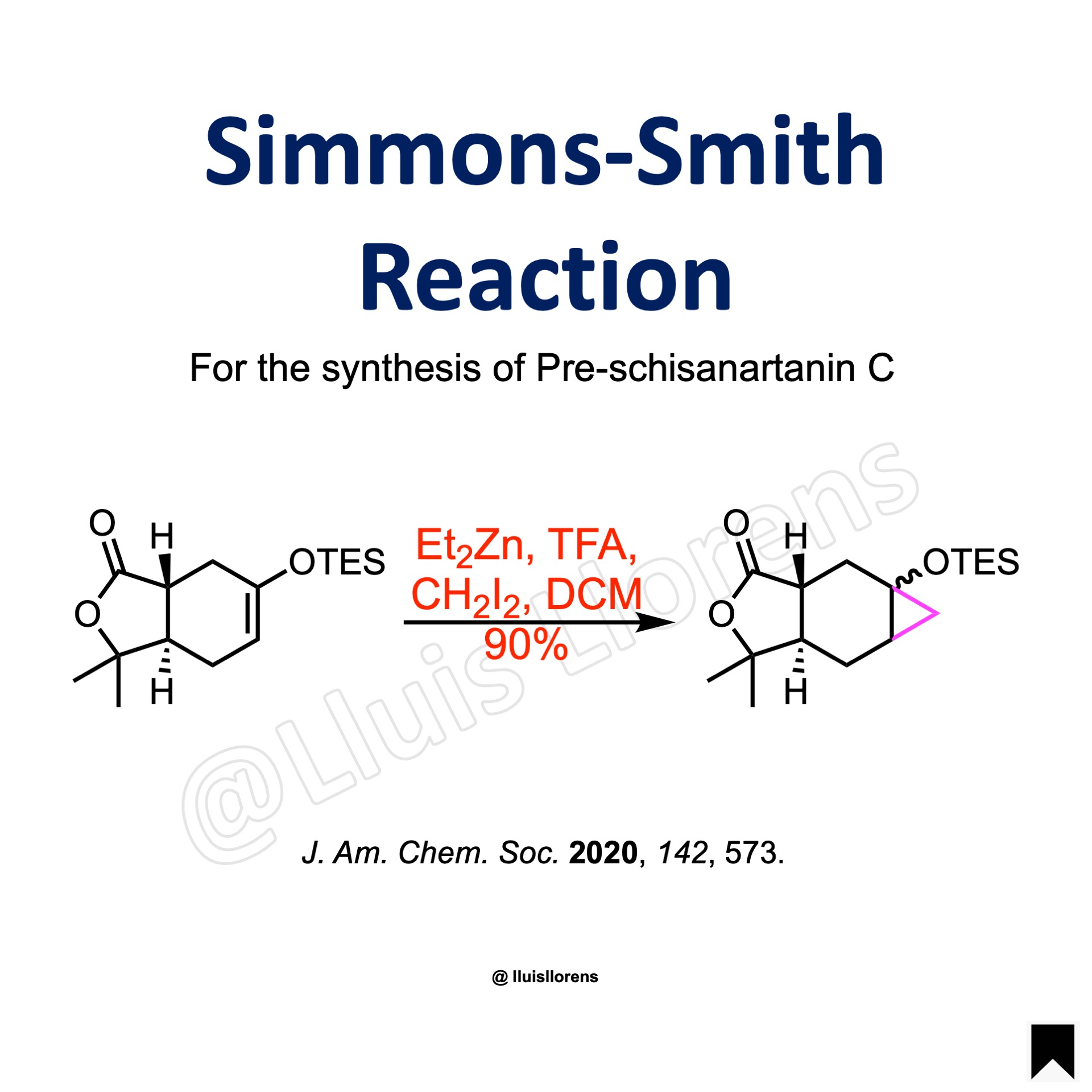
To a solution of CH2Cl2 (67 mL) was added a solution of Et2Zn (2.0 eq) under N2 atmosphere and cooled to 0 ºC, followed by addition of a solution of trifluoroacetic acid (2.0 eq) in CH2Cl2 (135 mL) in a dropwise manner. (CAUTION: due to the production of a lot of white smoke, the reaction needs to be carefully controlled to prevent the material sprayed), and the resultant white slurry was then stirred vigorously at room temperature for another 2 h, until no more bubbles released. To this solution was added a solution of CH2I2 (2.0 eq) in CH2Cl2 (30 mL) in a dropwise manner at –10 ºC, and the mixture was stirred at the same temperature until the mixture became clear. To this solution was added a solution of the alkene (67.3 mmol, 1.0 eq) in CH2Cl2 (105 mL) at –10 ºC, and the resultant mixture was then warmed to room temperature slowly, and stirred at the same temperature for 12 h. The reaction was quenched by pouring into a solution of NaHCO3 and Na2EDTA, followed by addition of a solution of NH4Cl to dissolve the precipitation. The organic phase was separated, and the water phase was extracted with CH2Cl2. The combined organic phases were concentrated, and the residue was purified by a flash column chromatography to give the product in 90% yield as mixture of two diastereomers.
Learn More Named Reactions
[instagram-feed feed=2]