Shapiro Olefination
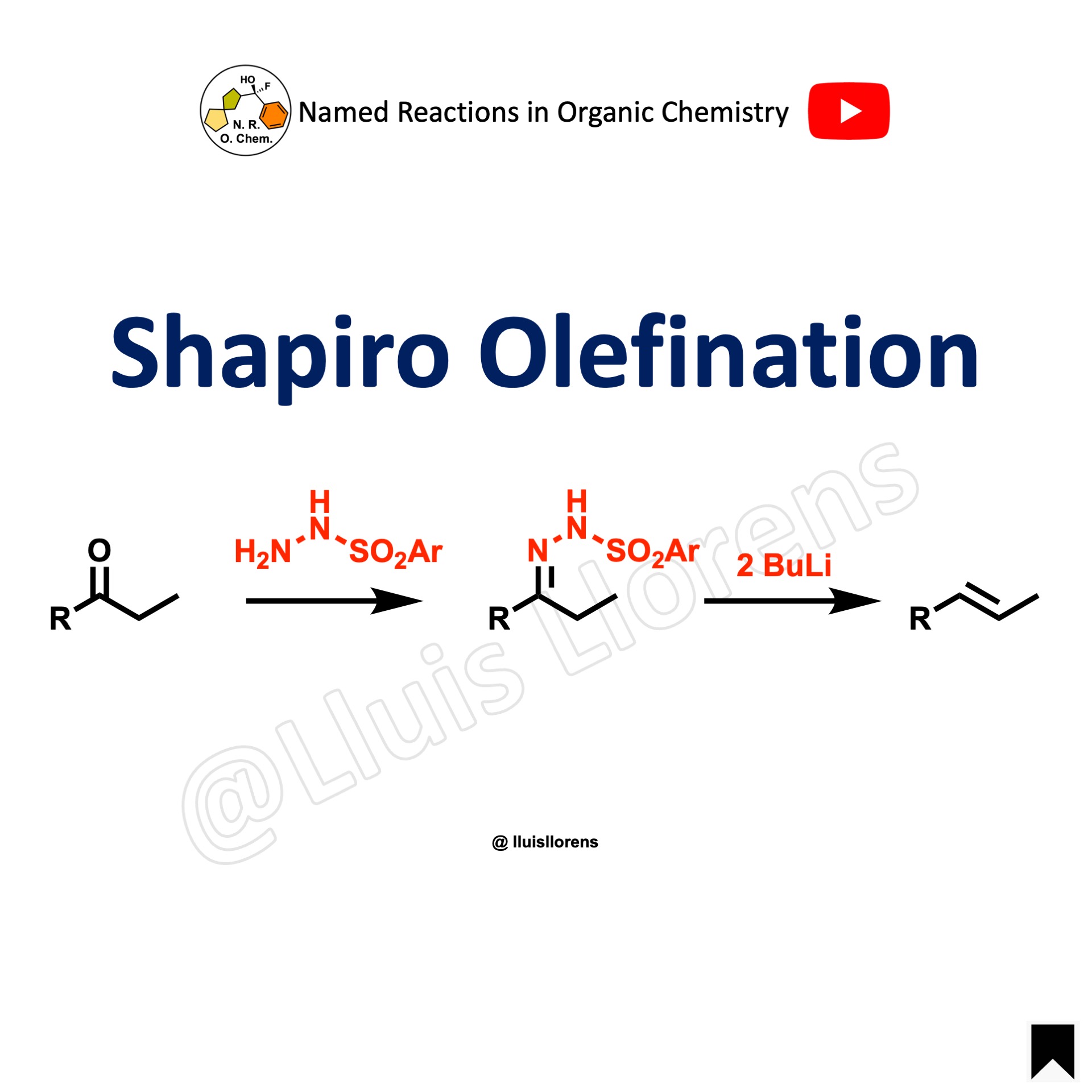
The Shapiro olefination allows the preparation of alkenes from the corresponding aldehydes or ketones. The reaction involves an intermediate hydrazone, and it is carried out in the presence of 2 equivalents of an organolithium reagent.
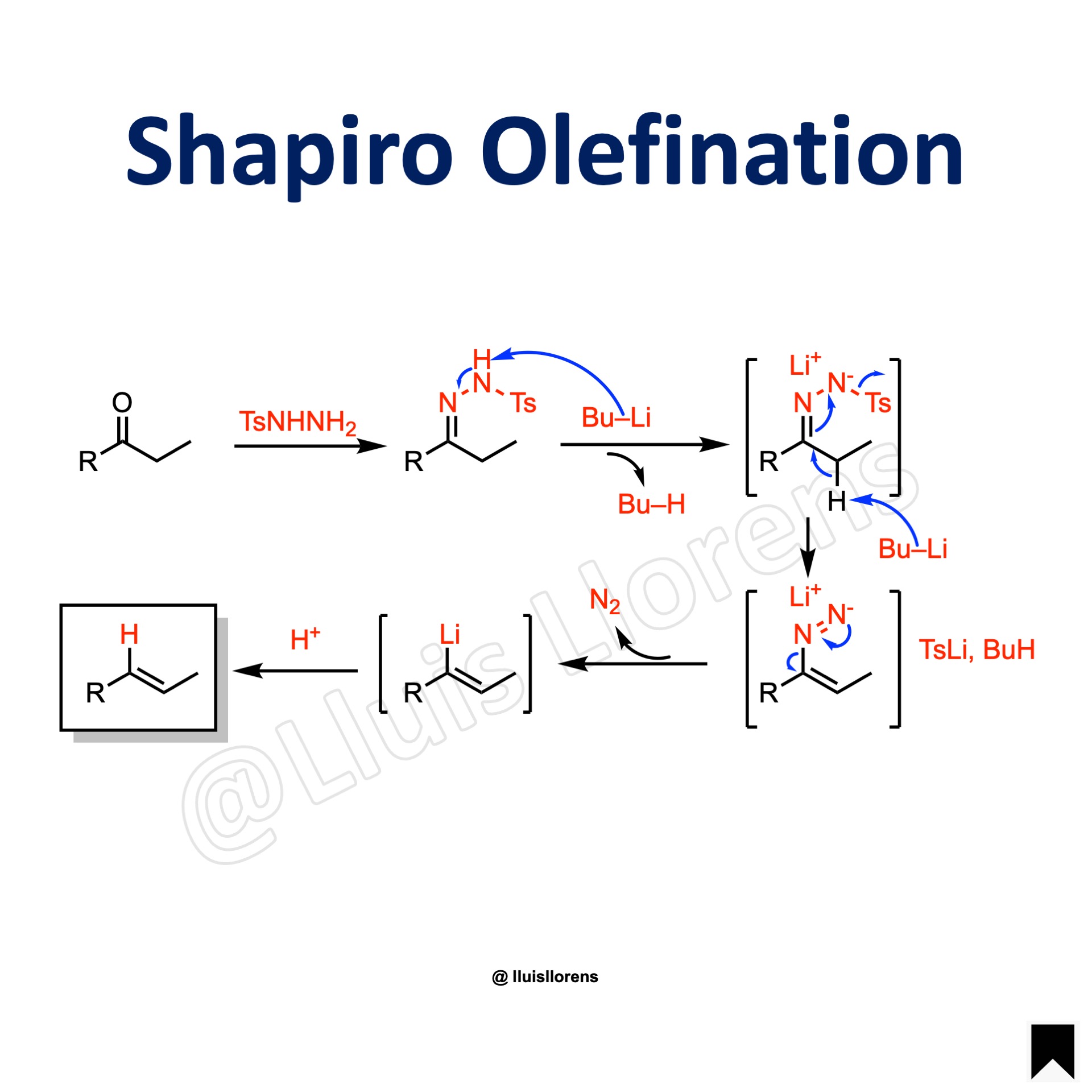
Reaction Mechanism
1. The ketone reacts with p-toluensulfonylhydrazide to form a tosylhydrazone. 2. The strong base abstracts the proton from the hydrazone. Then, a second equivalent of the base abstracts the less acidic proton alpha to the hydrazone carbon. 3. The tosyl group is eliminated producing a diazonium ion. 4. The anion loses nitrogen resulting in a vinyllithium species that can react with various electrophiles.
Example
Experimental Procedure
To a solution of the ketone (0.59 mmol, 1.0 equiv) in THF (4 mL) at room temperature was added 4-methylbenzenesulfonhydrazide (1.0 equiv), followed by a catalytic amount (ca. 7 mg) of PPTS, the mixture was stirred at 25 °C for 12 h. The reaction was quenched with water and extracted with EtOAc. The combined organic layers were washed with brine, dried over Na2SO4, filtered and concentrated under reduced pressure to give the crude product. This was immediately dissolved in dry THF (4 mL) under argon. To the solution, at 0 °C, was added 2.5 M solution of n–BuLi in Hexane (3.0 equiv) in portions via syringe. The mixture was stirred for 5 h with gradual warming to room temperature and sat. aq. NH4Cl (5 mL) was added. The aqueous layers were extracted with EtOAc, and the combined organic layers were washed with brine, dried, filtered and concentrated under reduced pressure. The residue was chromatographed on silica gel to give the allyl alcohol (60% yield).
Learn More Named Reactions
[instagram-feed feed=2]