Seyferth-Gilbert Homologation
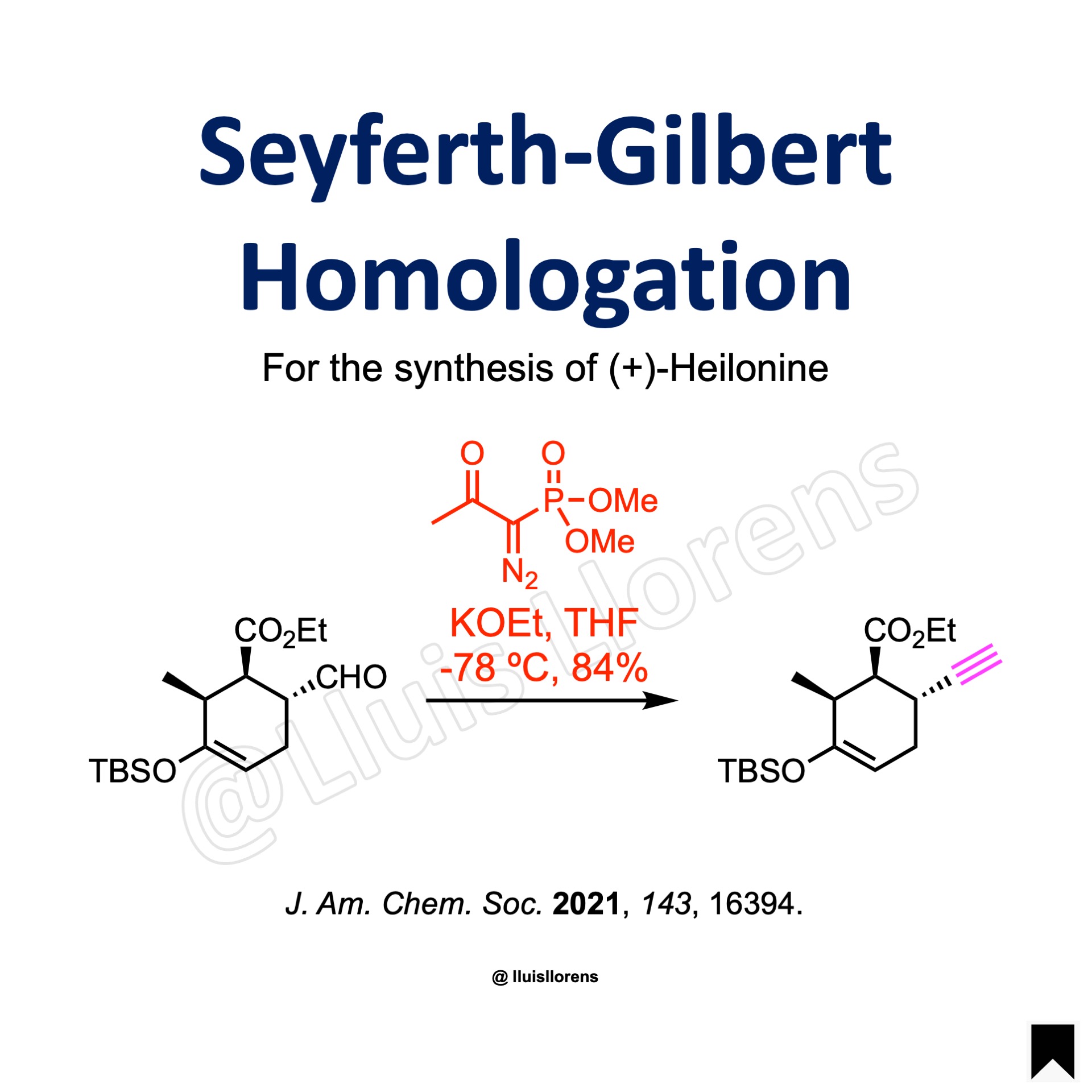
The Seyferth-Gilbert homologation allows the conversion of carbonyl compounds to the corresponding terminal or internal alkynes with the aid of alpha-diazophosphonates under basic conditions.
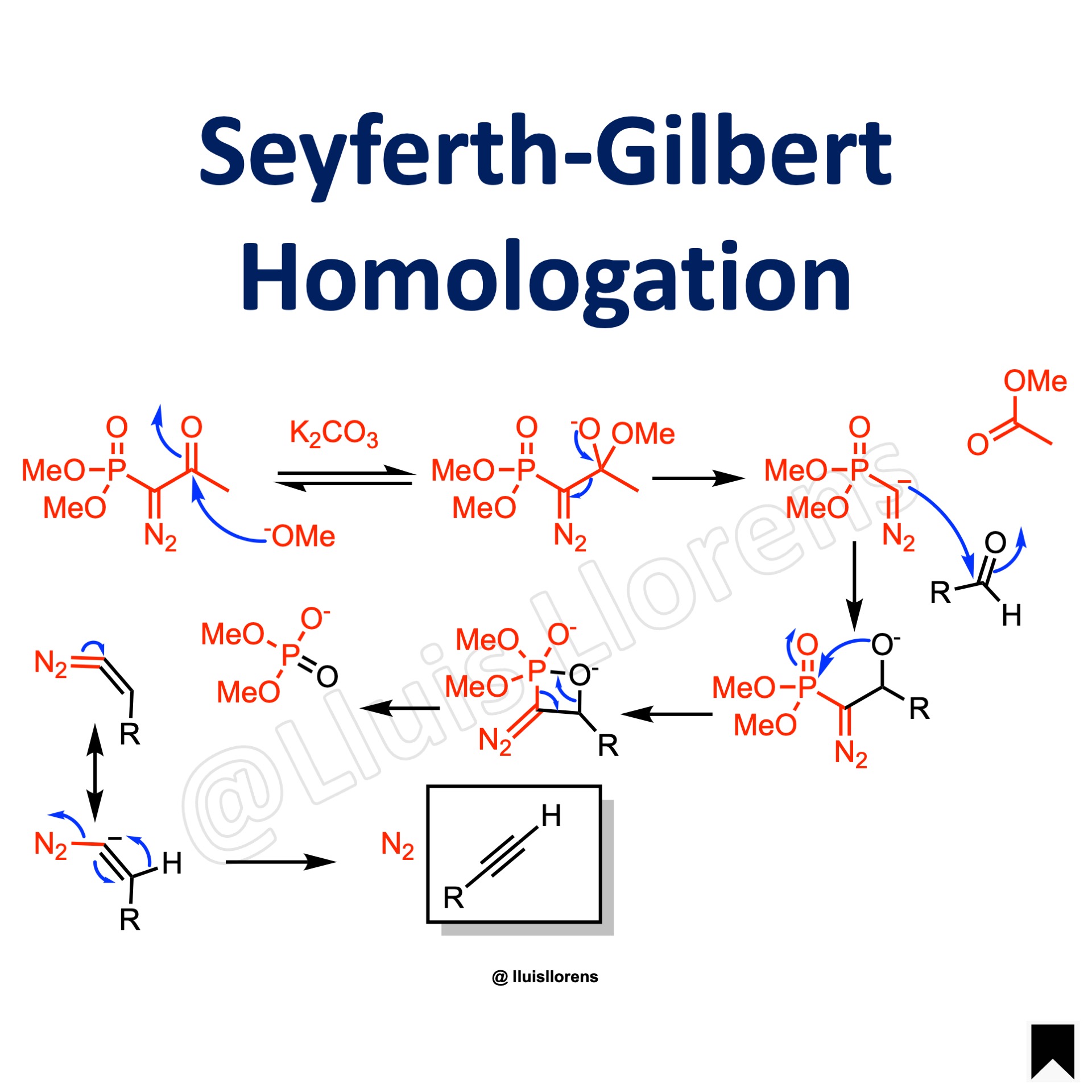
Reaction Mechanism
1. Deacylation of the Ohira-Bestman reagent by a mehoxide group. 2. The resulting carbanion attacks the carbonyl group of the aldehyde. 3. The oxaphosphetane-type intermediate breaks down to afford a diazoalkene. 4. The diazoalkene loses dinitrogen in an alpha-elimination reaction while the alkylidene carbene undergoes 1,2-shift to give the corresponding alkyne.
Experimental Procedure
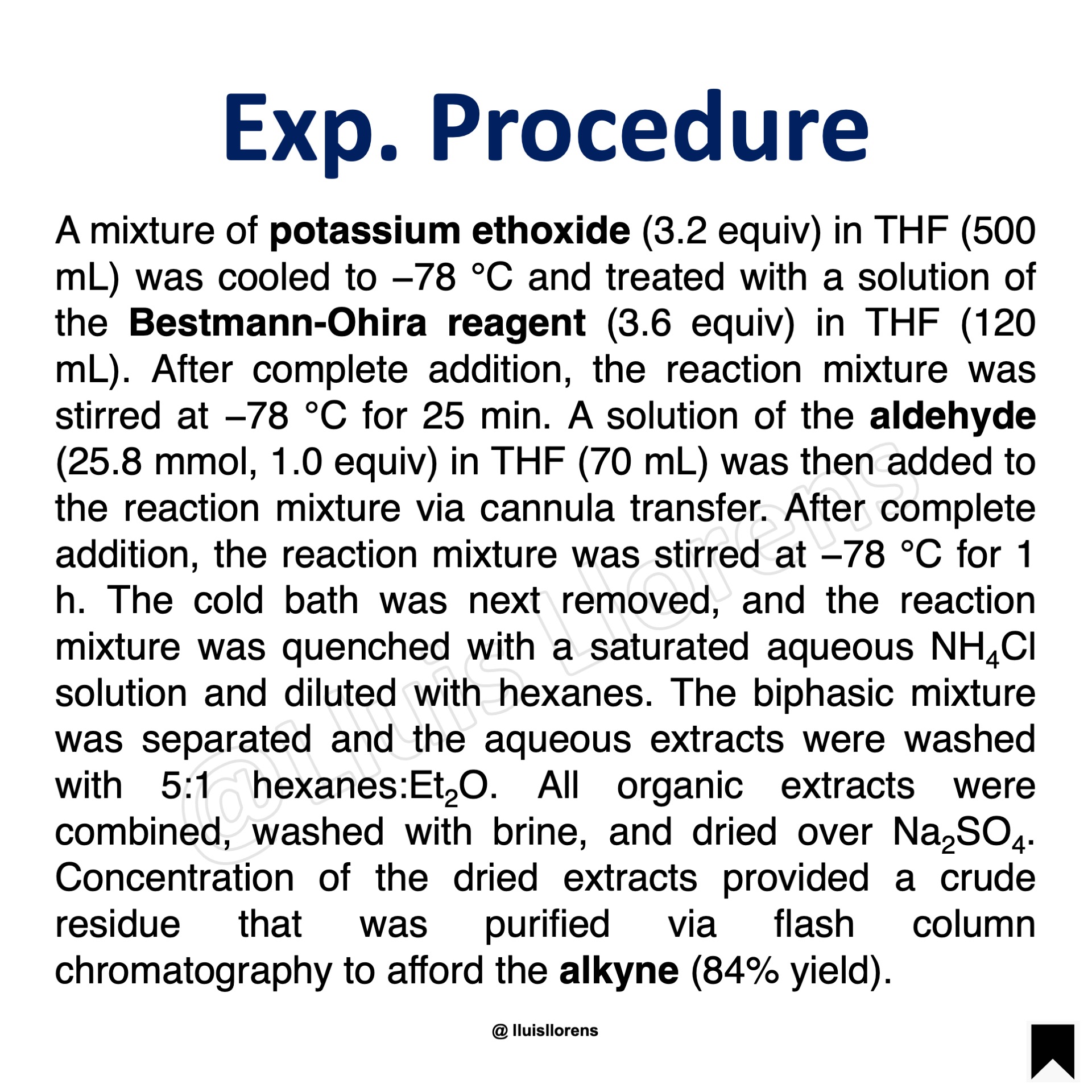
A mixture of potassium ethoxide (3.2 equiv) in THF (500 mL) was cooled to −78 °C and treated with a solution of the Bestmann-Ohira reagent (3.6 equiv) in THF (120 mL). After complete addition, the reaction mixture was stirred at −78 °C for 25 min. A solution of the aldehyde (25.8 mmol, 1.0 equiv) in THF (70 mL) was then added to the reaction mixture via cannula transfer. After complete addition, the reaction mixture was stirred at −78 °C for 1 h. The cold bath was next removed, and the reaction mixture was quenched with a saturated aqueous NH4Cl solution and diluted with hexanes. The biphasic mixture was separated and the aqueous extracts were washed with 5:1 hexanes:Et2O. All organic extracts were combined, washed with brine, and dried over Na2SO4. Concentration of the dried extracts provided a crude residue that was purified via flash column chromatography to afford the alkyne (84% yield).
Learn More Named Reactions
[instagram-feed feed=2]