Rubottom Oxidation
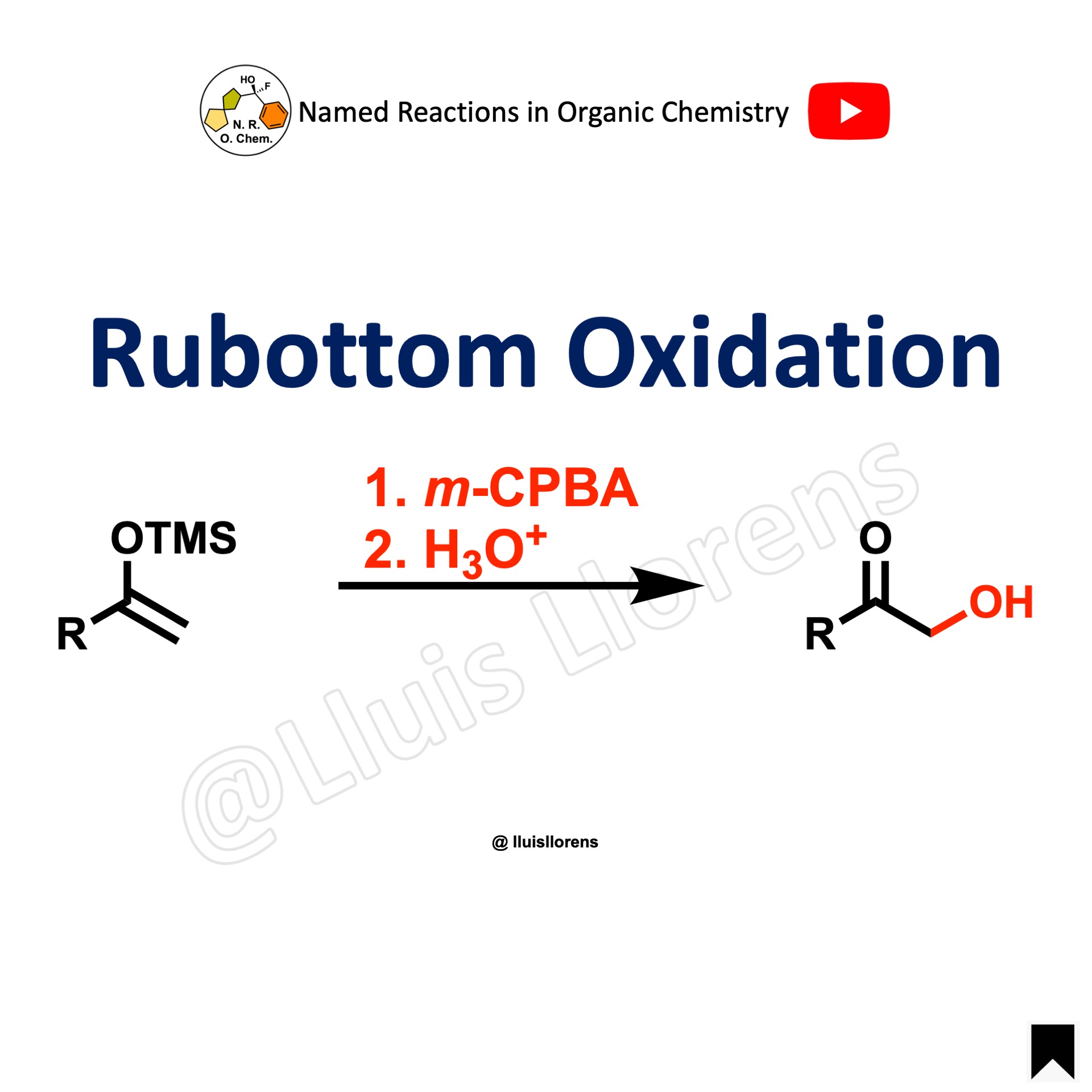
The Rubottom oxidation is the alpha-hydroxylation of carbonyl compounds by the reaction between silyl enol ethers and peroxyacids.
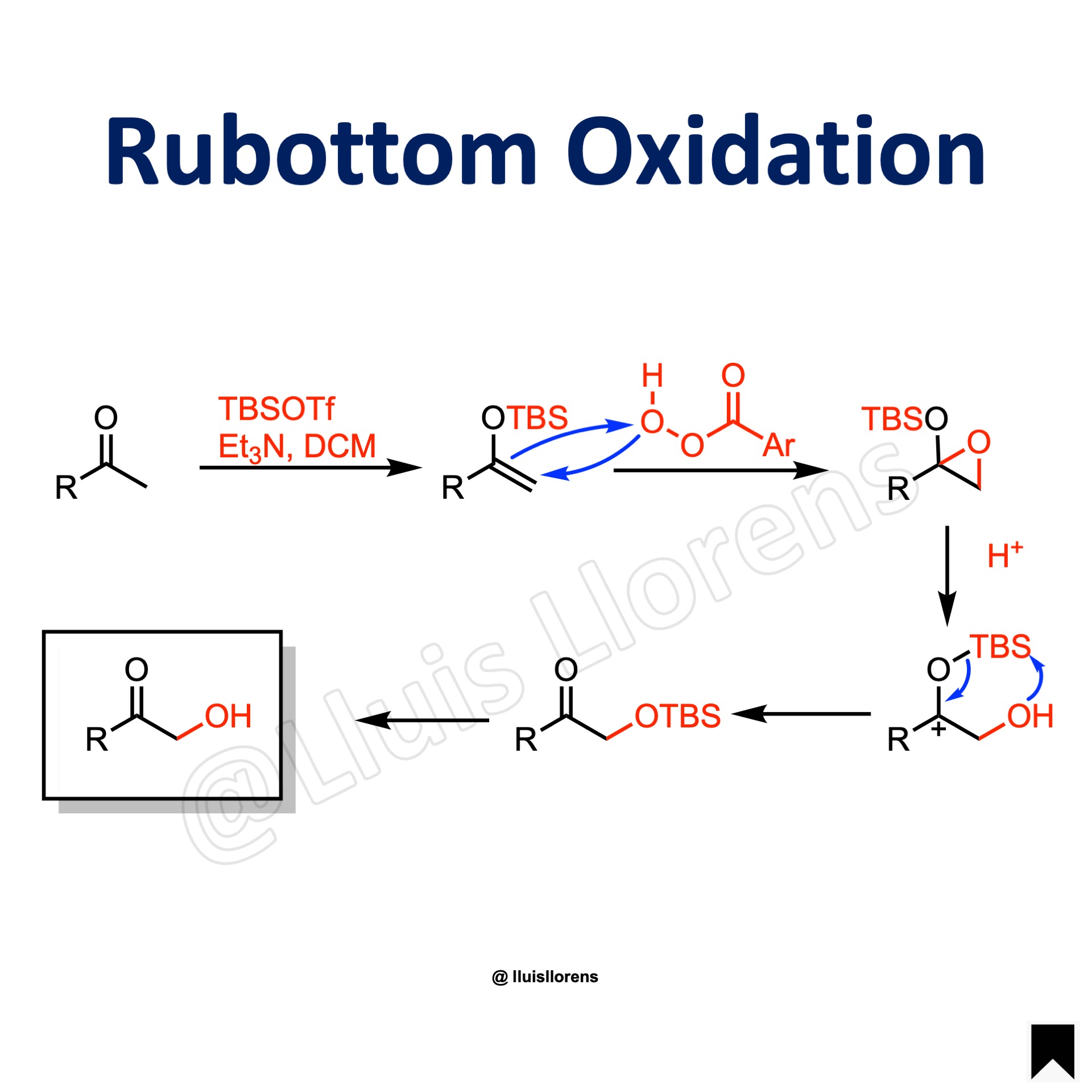
Reaction Mechanism
1. Formation of the silyl enol ether. 2. Oxidation of the silyl enol ether with the peroxyacid generates a silyloxy oxirane intermediate. 3. The epoxide ring opens under acidic conditions to yield an oxocarbenium ion. 4. The oxocarbenium ion undergoes 1,4-silyl migration to give a silyloxy ketone. (Brook Rearrangement.) 5. The silyloxy ketone is then hydrolyzed to the product.
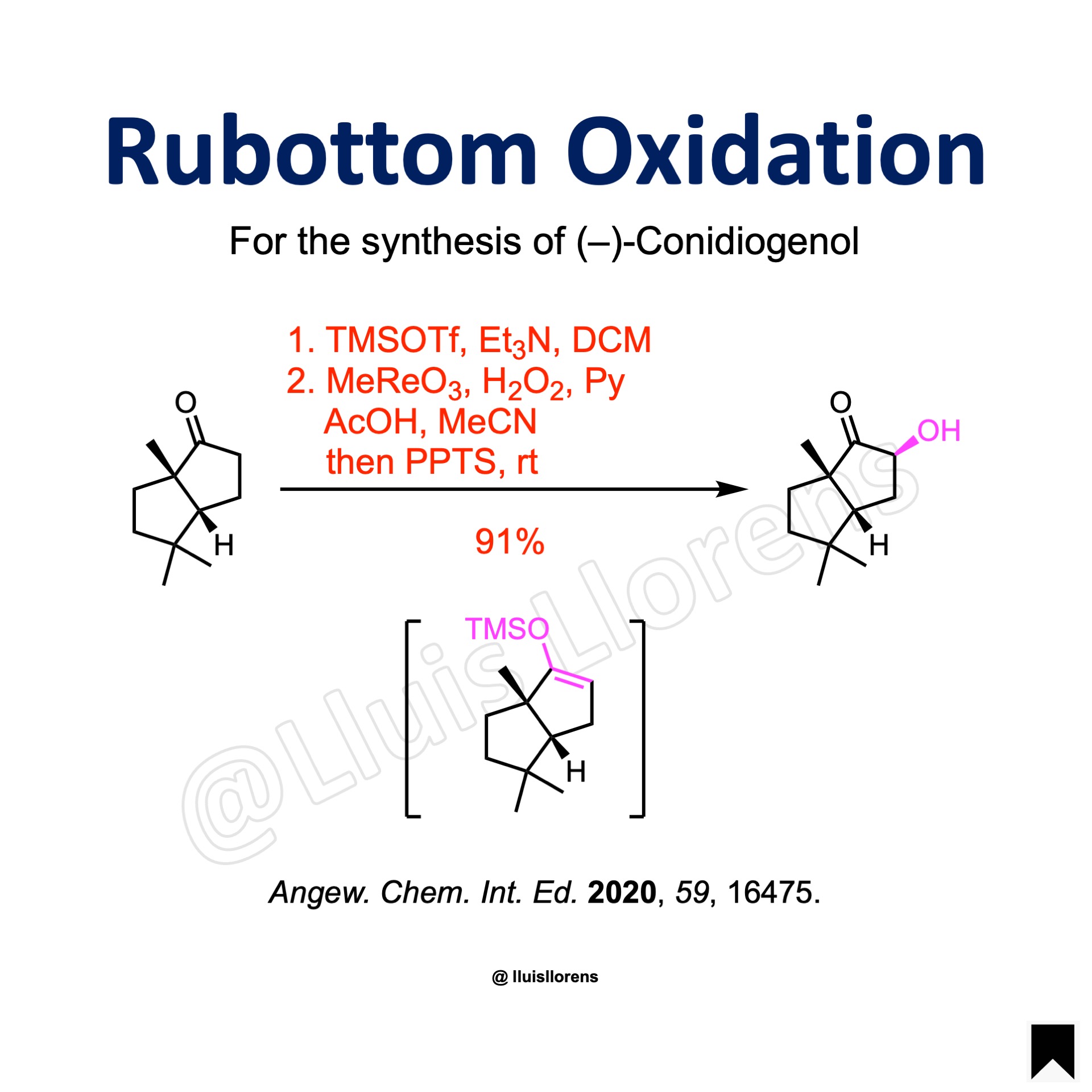
Experimental Procedure
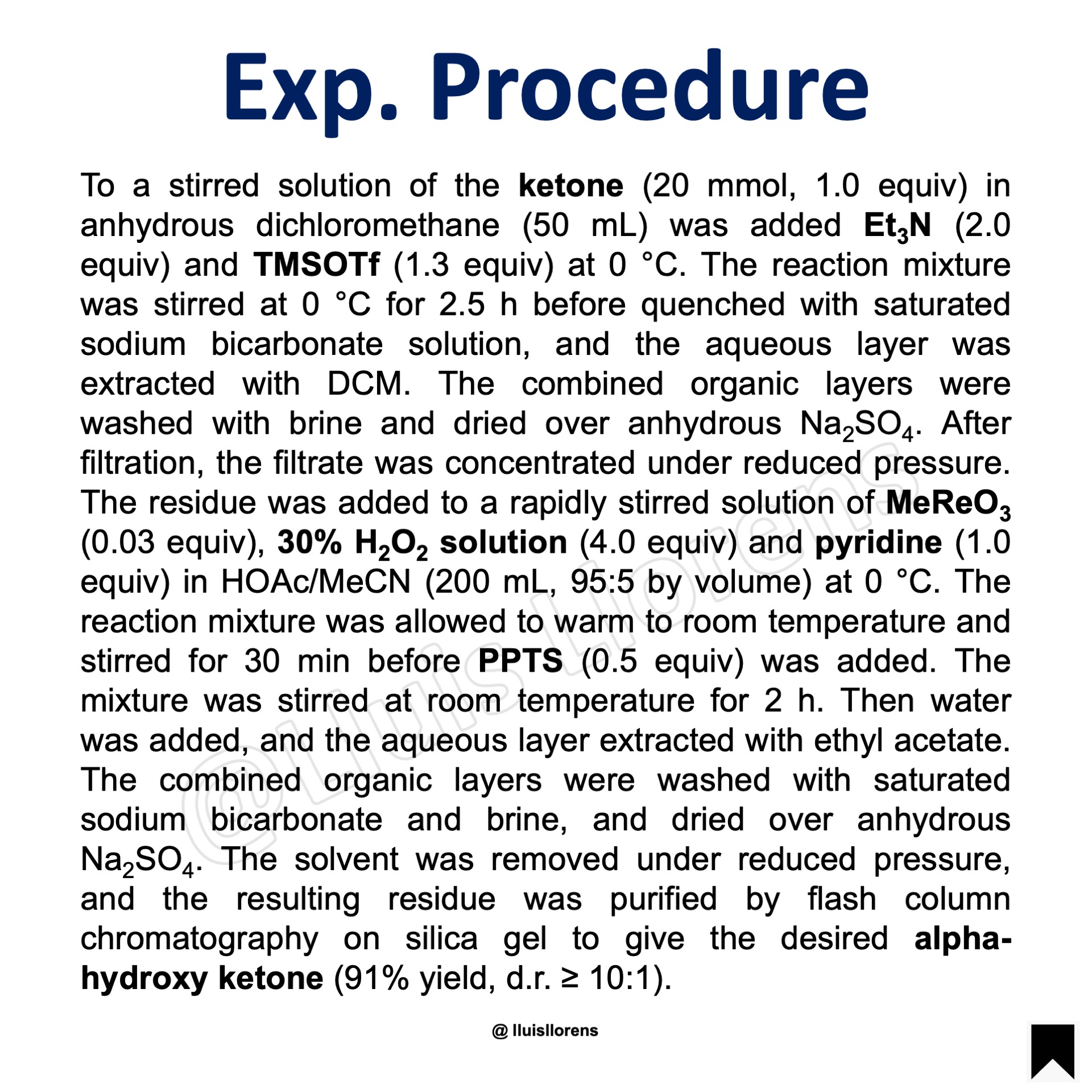
To a stirred solution of the ketone (20 mmol, 1.0 equiv) in anhydrous dichloromethane (50 mL) was added Et3N (2.0 equiv) and TMSOTf (1.3 equiv) at 0 °C. The reaction mixture was stirred at 0 °C for 2.5 h before quenched with saturated sodium bicarbonate solution, and the aqueous layer was extracted with DCM. The combined organic layers were washed with brine and dried over anhydrous Na2SO4. After filtration, the filtrate was concentrated under reduced pressure. The residue was added to a rapidly stirred solution of MeReO3 (0.03 equiv), 30% H2O2 solution (4.0 equiv) and pyridine (1.0 equiv) in HOAc/MeCN (200 mL, 95:5 by volume) at 0 °C. The reaction mixture was allowed to warm to room temperature and stirred for 30 min before PPTS (0.5 equiv) was added. The mixture was stirred at room temperature for 2 h. Then water was added, and the aqueous layer extracted with ethyl acetate. The combined organic layers were washed with saturated sodium bicarbonate and brine, and dried over anhydrous Na2SO4. The solvent was removed under reduced pressure, and the resulting residue was purified by flash column chromatography on silica gel to give the desired alpha-hydroxy ketone (91% yield, d.r. ≥ 10:1).
Learn More Named Reactions
[instagram-feed feed=2]