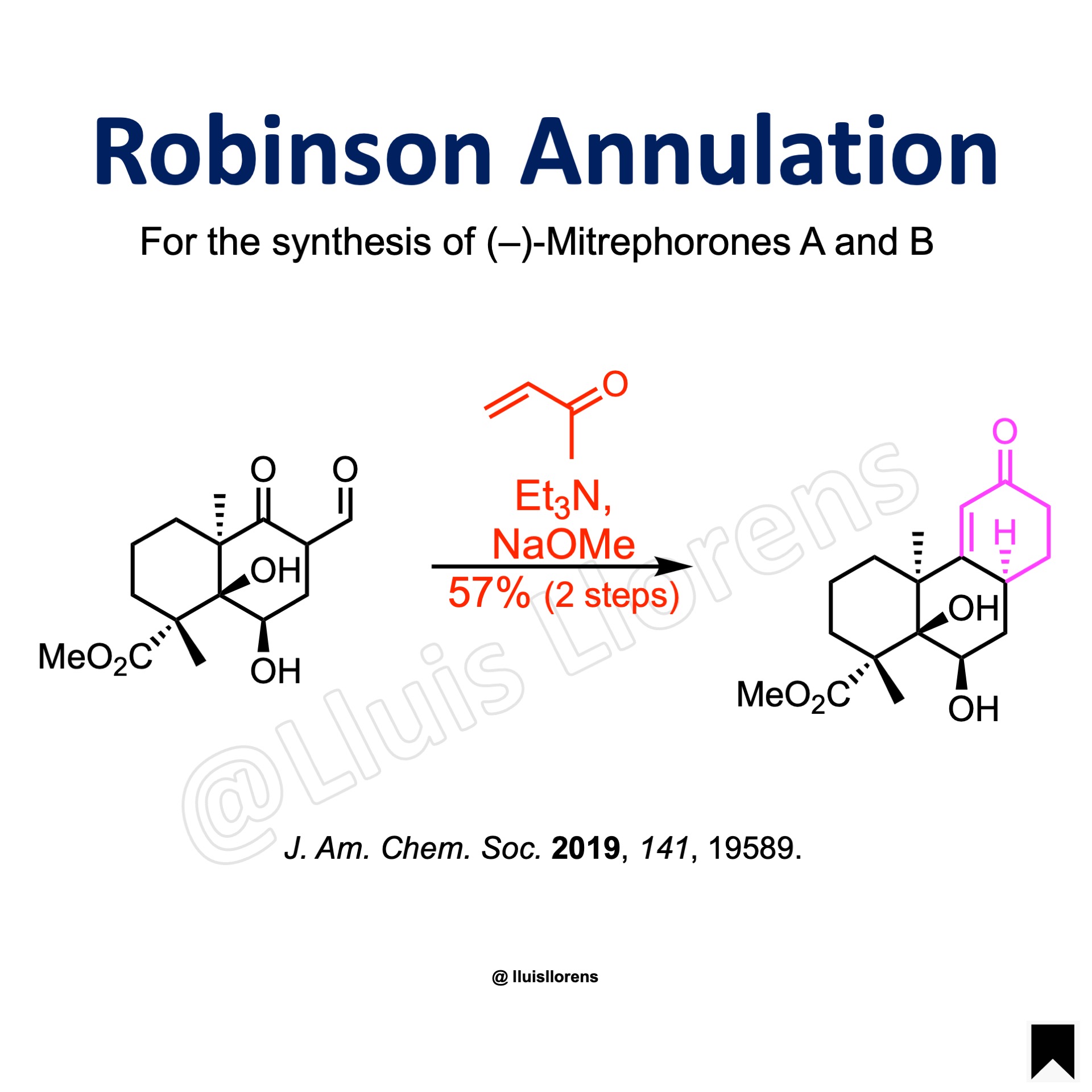
Robinson Annulation
The Robinson annulation is a C–C bond-forming reaction for the synthesis of six-membered rings. It combines a Michael addition followed by an aldol condensation. It is used in organic chemistry for ring formation to create 6 membered rings. For example, the reaction of a ketone such as cyclohexanone with an α,β-unsaturated ketone such as methyl vinyl ketone to give the corresponding bicyclic enone.
General features:
1. It can be both acid- and base-catalyzed. 2. It is a one-pot reaction; however, higher yields are usually obtained when the Michael adduct is isolated and then subjected to the aldol reaction.
Reaction Mechanism
1. Formation of the enolate by a base. 2. Conjugate addition reaction between the nucleophile and the Michael acceptor. 3. Proton abstraction from the protonated base. 4. Formation of the enolate by a base. 5. Direct addition of the nucleophile to the carbonyl carbon. 6. Final dehydration leads to the annulation product.
Experimental Procedure
To a solution of the aldehyde (1.0 eq) in DCM were added triethylamine (4.0 eq) and freshly distilled methyl vinyl ketone (5.0 eq) at 23 °C. The mixture was stirred for 96 h under exclusion of light. The mixture was then concentrated to yield the crude Michael adduct. The Michael adduct was dissolved in MeOH, and sodium methoxide (3.0 eq) was added in one portion while stirring at 23 °C. After 24 h, a further portion of sodium methoxide (1.0 eq) was added. After further 20 h, saturated aqueous ammonium chloride solution was added. The layers were separated, and the aqueous layer was extracted with DCM. The combined organic layers were washed with saturated aqueous sodium chloride solution, dried over magnesium sulfate, filtered, and concentrated. The residue was then purified by flash column chromatography on silica gel to yield the annulation-deformylation product.
Learn More Named Reactions
[instagram-feed feed=2]