Reduction Reactions
This post is about reduction reactions in organic chemistry. These are reactions that change the oxidation state of the atoms on the substrate. Thus, reduction occurs when the oxidation number of an atom becomes smaller. The scope of this post is limited to the reduction of the carbon atom. The post is active and growing, so you can expect more examples to be added over time. Please be sure to come back. The updates will be announced on my instagram account.
Last update 23/12/16.
Deoxygenation Reactions
Chemical reactions involving the removal of oxygen atoms from a molecule.
5. For the synthesis of Ambiguine G.
The hydroxyl group was removed under ionic hydrogenation conditions (BF3·OEt2 and Et3SiH), forming a single diastereomer.
J. Am. Chem. Soc. 2021, 143, 10872. Open access.
4. For the synthesis of Aberrarone.
Barton-McCombie reaction: The hydroxy-functional group in an organic compound is replaced by a hydrogen atom to give an alkyl group. The alcohol is first transformed into a thioxoester that is then exposed to n-Bu3SnH and AIBN in refluxing toluene.
3. For the synthesis of Sotagliflozin.
Triethylsilyl hydride reduction of the ketone.
2. For the synthesis of Pexidartinib.
The alcohol was reduced with triethylsilane. Subsequent treatment with TFA removed both Boc protecting groups to afford the aminopyridine.
1. For the synthesis of Cephanolide A.
A Barton-McCombie deoxygenation reaction.
Reduction of Alkenes and Alkynes
Reactions where an alkyne or alkene is reduced to the corresponding alkene or alkane.
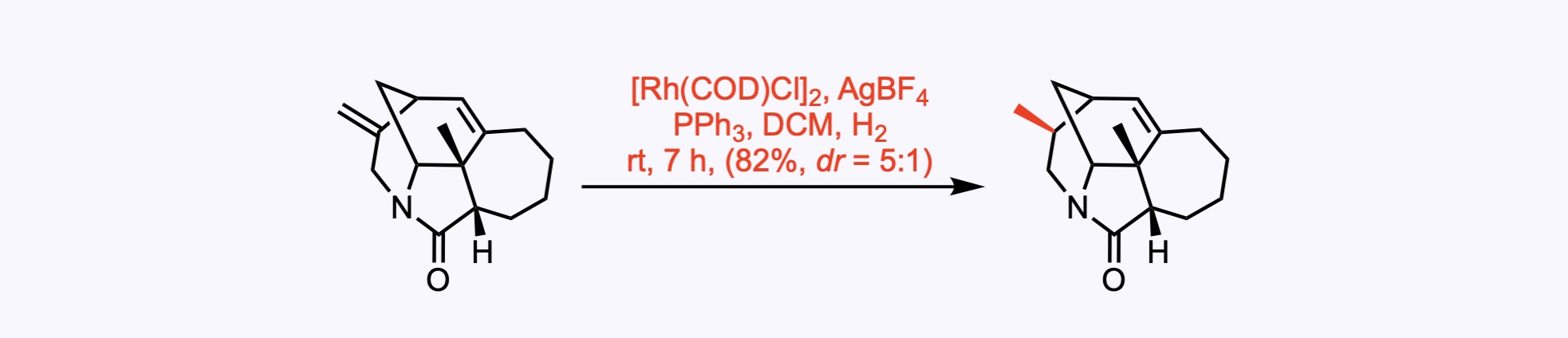
8. For the synthesis of Calyciphylline R.
The 1,1-disubstituted alkene in the substrate was diastereoselectively hydrogenated (dr = 5:1) under Li’s conditions.
7. For the synthesis of Longiborneol.
This is an interesting example of a vinyl sulfonate being used as a radical acceptor in metal-hydride hydrogen atom transfer, MHAT, chemistry.
Subjecting the substrate to optimized Fe-HAT conditions, which required buffering agents, smoothly effected cyclization in a remarkable 85% yield. Presumably, this reaction occurs by H-atom transfer from an iron hydride to the terminal position of the western alkene of the substrate, generating a tertiary radical that adds into the vinyl phenyl sulfonate, leading to a homolytic cleavage of the S–O bond to give the ketone.
6. For the synthesis of Asnovolin A.
Stereoselective metal hydride atom transfer, MHAT, reduction to install an axial methyl group under Baran’s conditions [Fe(acac)3, PhSiH3, EtOH]. In initial experiments, hydrogenation (H2, Pd/C) resulted in full recovery of starting material. Alternatively, MHAT hydrogenation of alkenes pioneered by Shenvi and co-workers has been widely applied in natural product synthesis to afford thermodynamically stable products. This is a rare example of production of the thermodynamically less stable axial methyl group through 1,6-MHAT, where an initially formed tertiary radical abstracts a spatially proximal hydrogen atom through an interesting chair-to-boat conformational change under Curtin−Hammett conditions.
5. For the synthesis of Chimonanthine.
Hydrogenation using Zn and AcOH.
4. For the synthesis of Amphidinolide C2.
Hydrogenation using Lindlar’s catalyst. The Lindlar catalyst is a heterogeneous catalyst that is used for the selective hydrogenation of alkynes to alkenes, allowing the selective hydrogenation of triple bonds to cis-double bonds.
3. For the synthesis of Drupacine.
Hydrogenation using Adam’s catalyst.
2. For the synthesis of Cephanolide A.
Hydrogenation and susbsequent epimerization. An epimerization is a process in which there is a change in the configuration of only one chiral center. As a result, a diastereomer is formed. In this case, the methyl group resulting from hydrogenation epimerizes after being exposed to DBU.
1. For the synthesis of Adociaquinone A.
Birch reduction: the 1,4-reduction of aromatic rings to the corresponding unconjugated cyclohexadienes and heterocycles by alkali metals (Na, Li, K) dissolved in liquid ammonia in the presence of alcohol.
Reduction of Carbonyl Compounds
10. For the synthesis of Incargranine A.
A chemoselective iridium-catalyzed reduction of an amide to produce a silylated hemiaminal (employing a modified Vaska’s complex (IrCl(CO)(PPh3)2) and 1,1,3,3-tetramethyldisiloxane (TMDS)). Upon acid treatment, the silylated hemiaminal undergoes elimination to generate the corresponding iminium ion, to which the pendant alcohol adds, yielding a cyclic hemiaminal. The desired reactivity is accomplished by directly treating the reaction mixture with dry hydrochloric acid in 1,4-dioxane. This single step achieves two critical objectives: deprotection of the tri-n-butylsilyl (TBS) group and initiation of a retro-oxa-Mannich/oxa-Michael/Mannich cascade, along with concurrent p-methoxybenzyl (PMB) deprotection.
9. For the synthesis of Amphidinolide F.
The high syn selectivity could be explained by the bulkiness of the silane reagent in a Felkin−Ahn type model, with Ph3SiH being noticeably superior to Et3SiH.
8. For the synthesis of Calyciphylline R.
Selective amide reduction conditions using Vaska’s complex (IrCl(CO)(PPh3)2). This is typical Darron Dixon chemistry in which Vaska‘s catalyst hydrosilylates the amide to the hemiaminal, which can be further functionalized. In this case, it is simply reduced with STAB to give the amine.
7. For the synthesis of Lascufloxacin.
Reduction of the amide to the corresponding amine with borane.
6. For the synthesis of Drupacine.
Reduction of the lactam moiety.
5. For the synthesis of Peniciketal A.
4. For the synthesis of Bathymdiolamide A.
3. For the synthesis of Clionastatin A.
Corey-Bakshi-Shibata reduction: the chemical reaction in which an achiral ketone is enantioselectively reduced to produce the corresponding chiral alcohol by the influence of the CBS oxazaborolidine catalyst.
2. For the synthesis of Peyssonnoside A.
Luche reduction. The luche reduction is the selective organic reduction of α,β-unsaturated ketones to allylic alcohols with sodium borohydride (NaBH4) and lanthanide chlorides, mainly cerium(III) chloride (CeCl3), in methanol or ethanol.
J. Am. Chem. Soc. 2021, 143, 14083. Open access.
1. For the synthesis of Altemicidin.
Reduction of the Nitro Group
4. For the synthesis of Quizartinib Dihydrochloride.
Reduction of the nitro group to generate aniline.
3. For the synthesis of Mirogabalin besylate.
Reduction of the nitro group with Fe.
2. For the synthesis of Spiroindimicin A.
Reduction of the nitro group with Zn.
Chem. Sci. 2021, 12, 10388. Open access.
1. For the synthesis of Bauhinoxepin C.
The nitro group and the double bond of the oxepine were both reduced with hydrogenation to provide the amine.
Reduction Reactions
2. For the synthesis of 10,22-Dioxokopsane.
Reduction of the nitrile to the corresponding amine and condensation with the ketone.
1. For the synthesis of Annotinolide C.
Reduction of the nitrile to the corresponding aldehyde.
J. Am. Chem. Soc. 2021, 143, 11951. Open access.






























0 Comments