Protection of Alcohols
This post is about reactions involving the protection of alcohols in organic chemistry. The post is active and growing, so you can expect more examples to be added over time. Please be sure to come back. The updates will be announced on my instagram account.
Check out the post about protection of amines. >>>
Last update 24/03/27.
Acetyl
The acetyl group (Ac), with the chemical formula −COCH3, contains a methyl group (−CH3) single-bonded to a carbonyl (C=O), making it an acyl group. It is removed by acid or base.
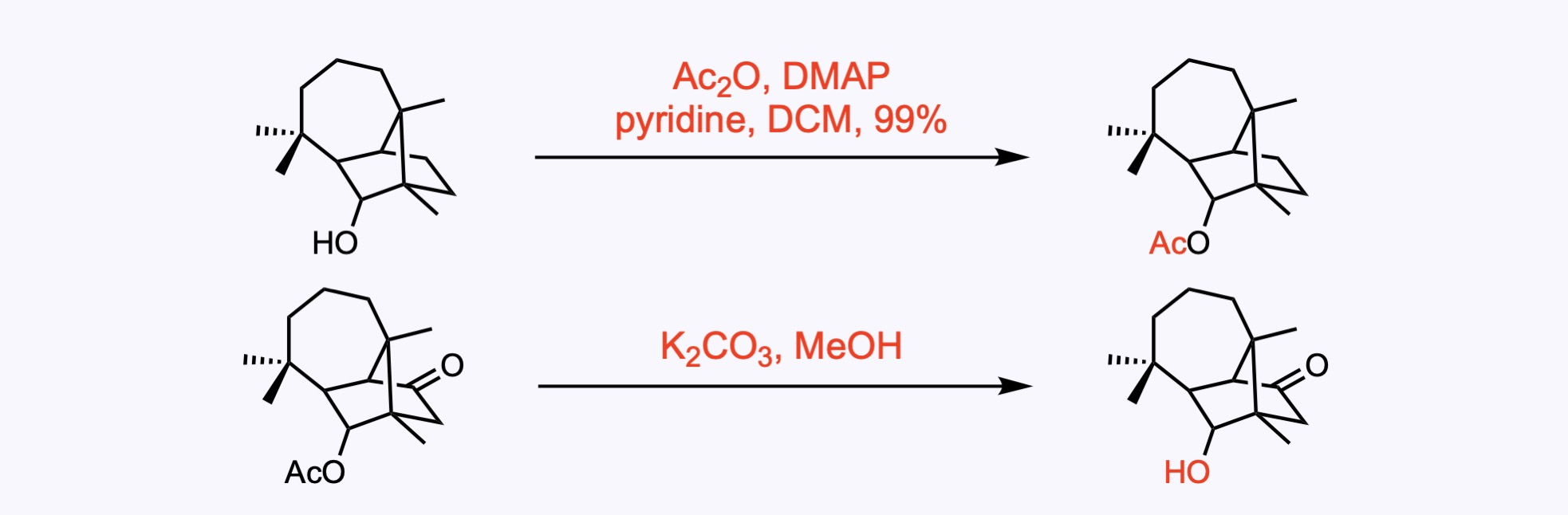
2. For the synthesis of Longiborneol.
1. For the synthesis of Clionastatin A.
Benzoyl
The benzoyl group (Bz), with the formula −COC6H5, is more stable than Ac group. It is also removed by acid or base.
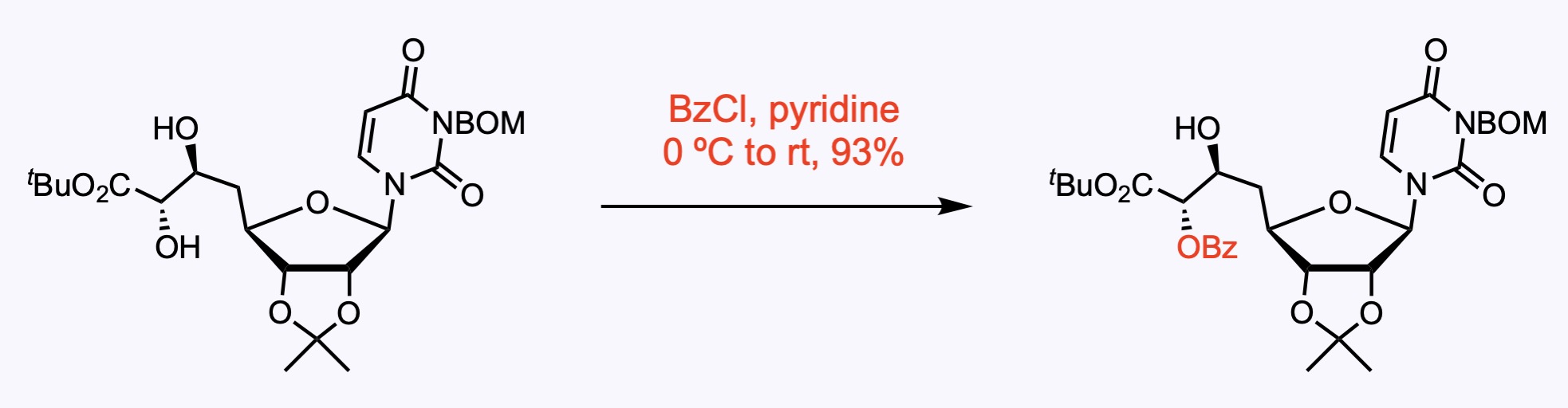
1. For the synthesis of A-94964.
Benzyl
The benzyl group (Bn) features a benzene ring (C6H6) attached to a methylene group (−CH2−) group. It is removed by hydrogenolysis.
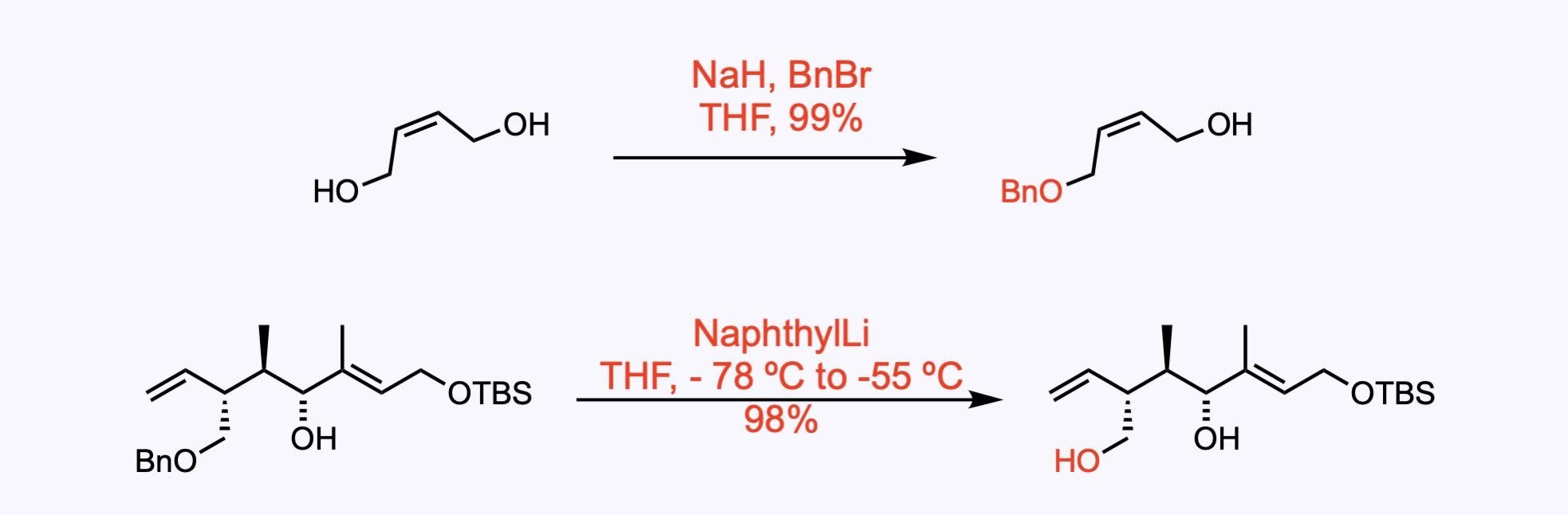
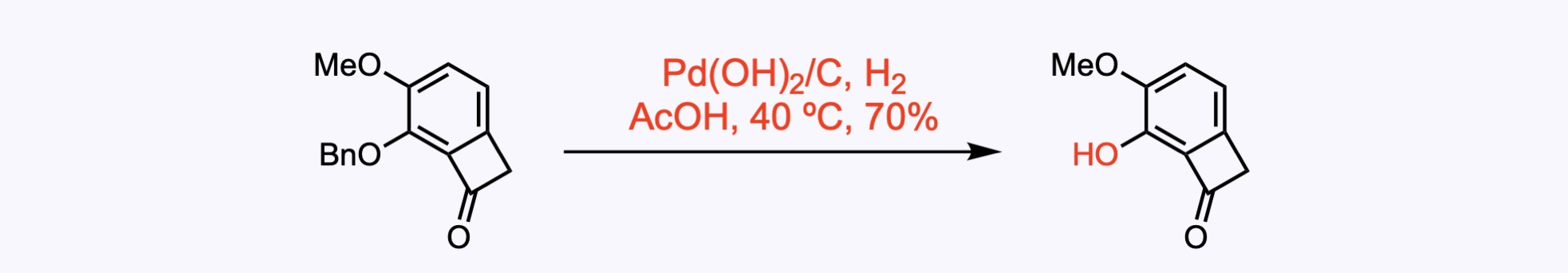
2. For the synthesis of Fusaequisin A.
(1) Monoprotection of the diol. (2) Reductive removal of the benzyl protecting group delivered the diol .
Chem. Eur. J. 2022, 28, e2021035. Open access.
1. For the synthesis of Thebainone A.
Benzyl deprotection via hydrogenation.
Methyl
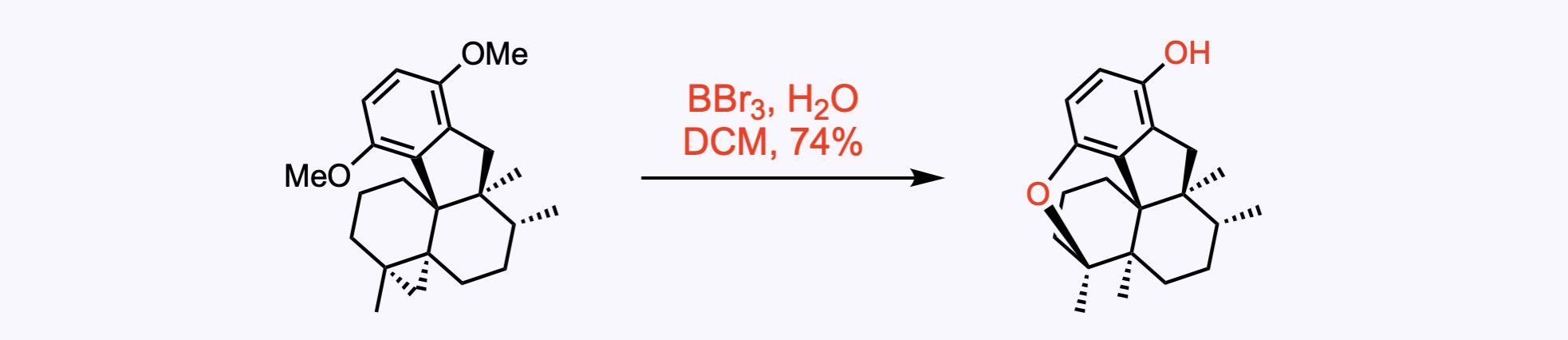
Methyl ethers – Cleavage is by TMSI in dichloromethane or acetonitrile or chloroform. An alternative method to cleave methyl ethers is BBr3 in DCM.
4. For the synthesis of (–)-Preussochromone D.
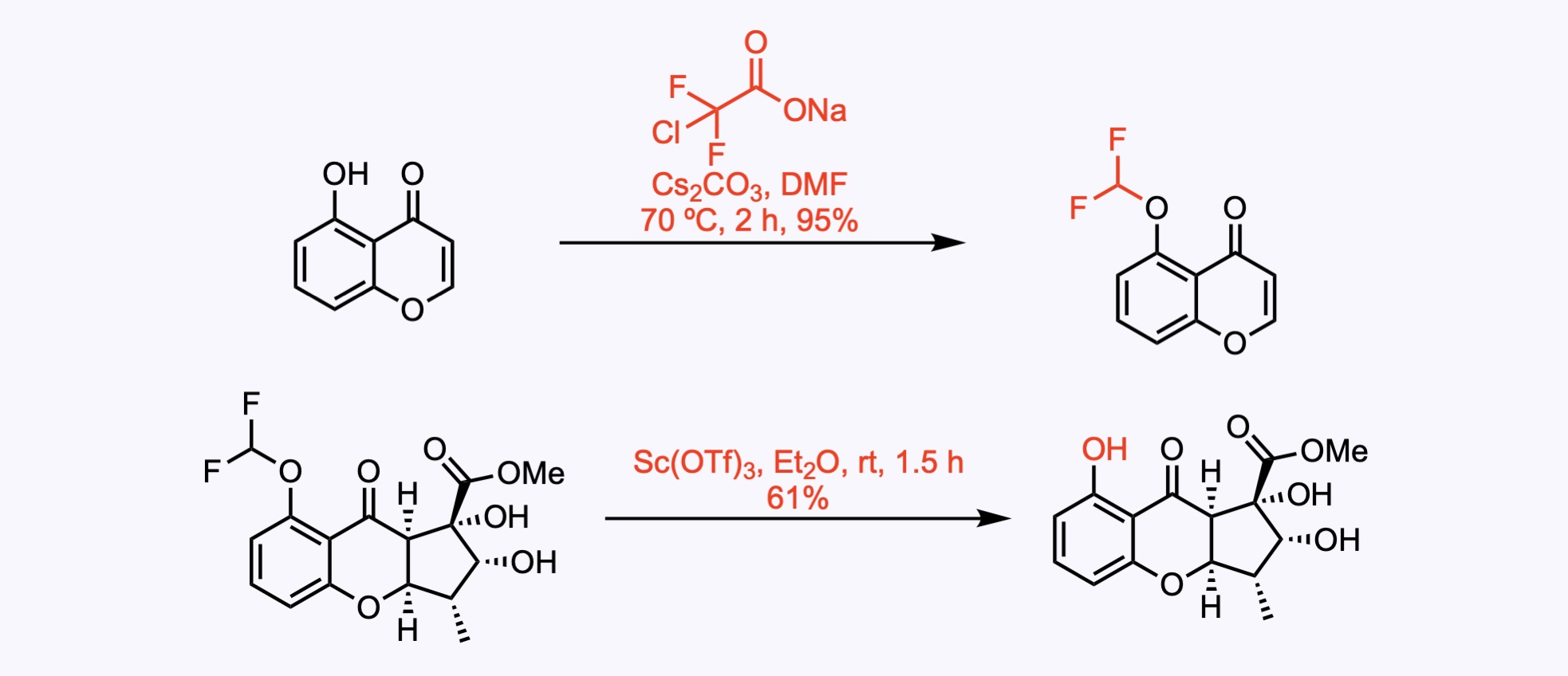
The substrate was alkylated with in-situ-generated difluorocarbene to give the corresponding difluoroether in 95% yield. This rather unusual electron-withdrawing protecting group was chosen to prevent side reactions of electron-rich arenes in the upfollowing oxidative steps. Deprotection of the phenol ether with scandium triflate gave the phenol in 61% yield.
3. For the synthesis of Cefiderocol.
2. For the synthesis of Dysiherbol A.
Angew. Chem. Int. Ed. 2021, 60, 14915. Open access.
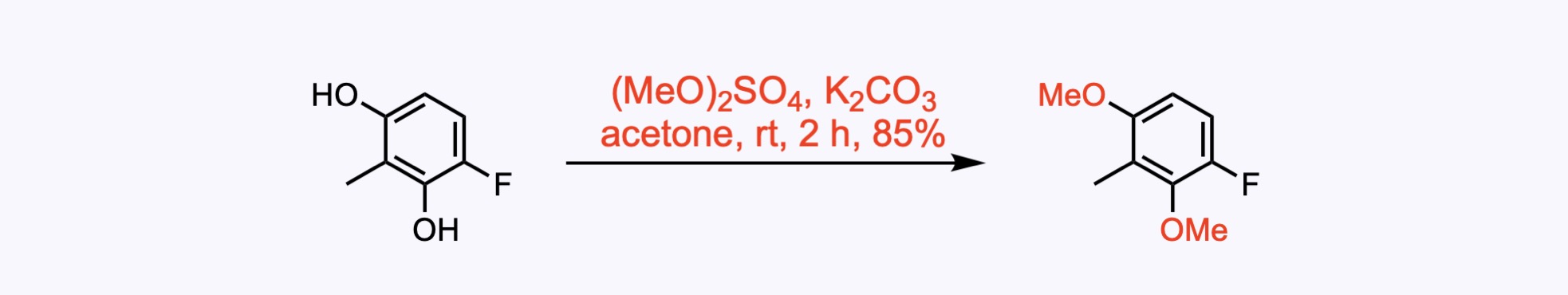
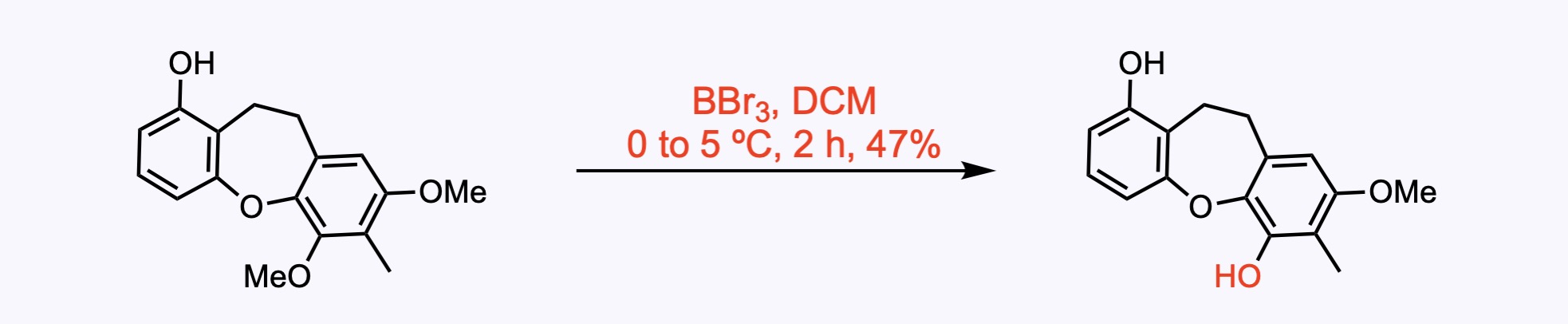
1. For the synthesis of Bauhinoxepin C.
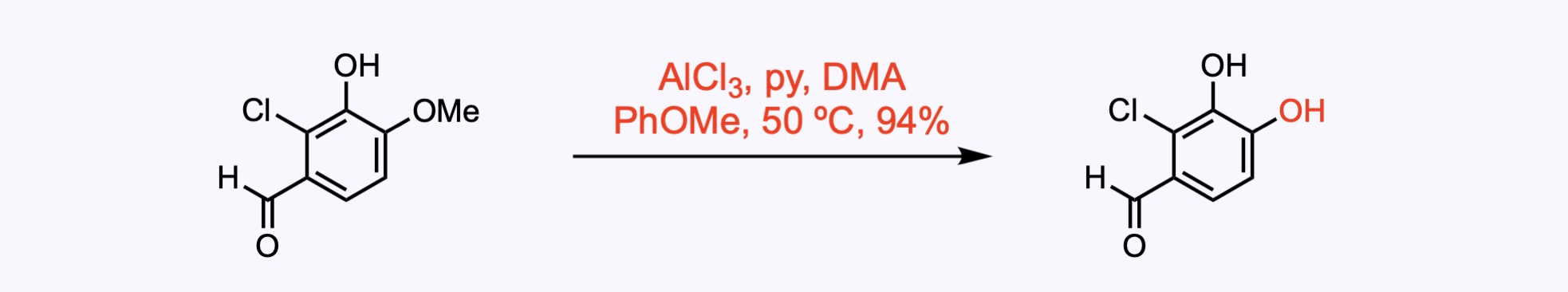
Both of the hydroxyl groups were methylated with dimethyl sulfate in the presence of potassium carbonate to provide dimethoxybenzene.
The methoxy group at the C6 position of the phenol was selectively demethylated with BBr3.
MOM
Methoxymethyl ether (MOM) – ROCH2OCH3 – Removed by acid.
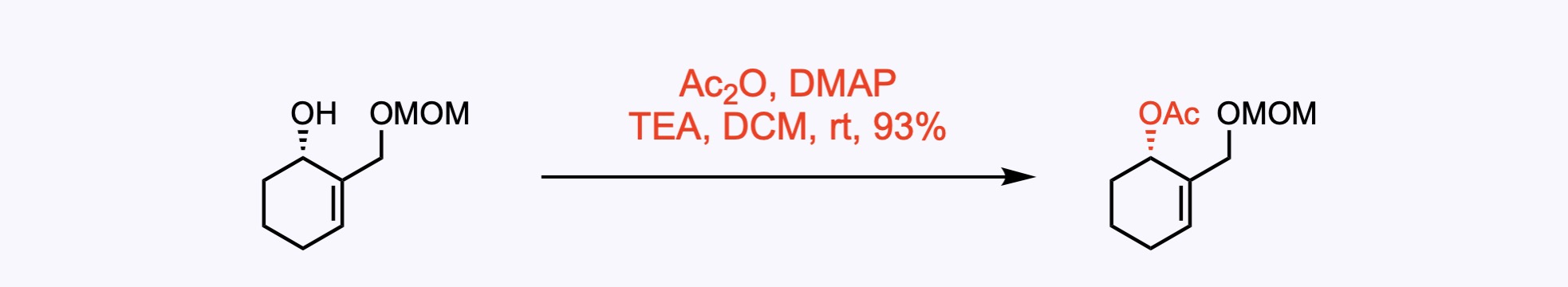
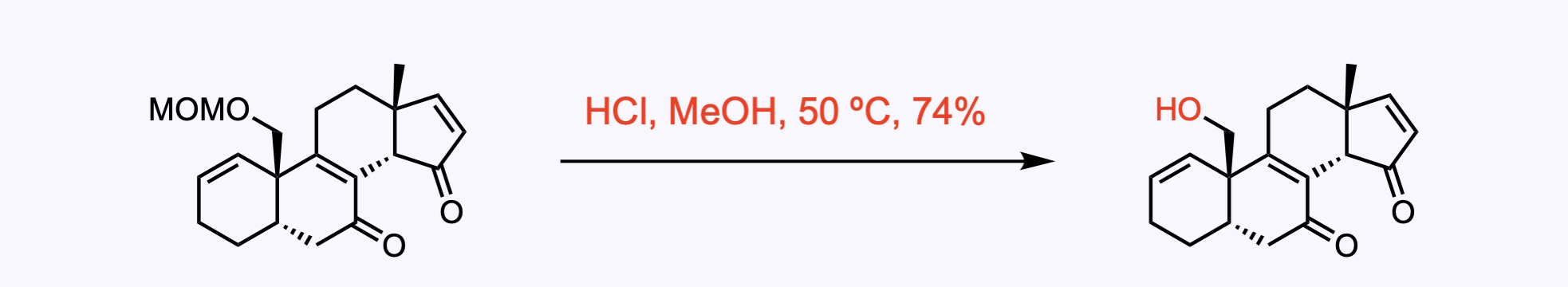
2. For the synthesis of Clionastatin A.
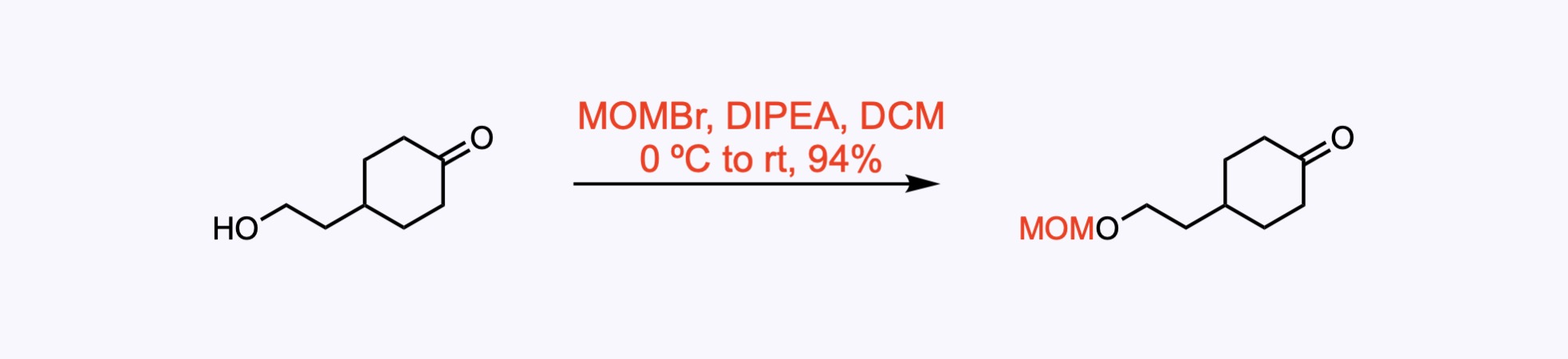
1. For the synthesis of Undulifoline.
Reaction of the readily available 4-(2-hydroxyethyl)cyclohexan-1-one with bromomethyl methyl ether under standard conditions afforded the MOM ether in 94% yield.
PMB
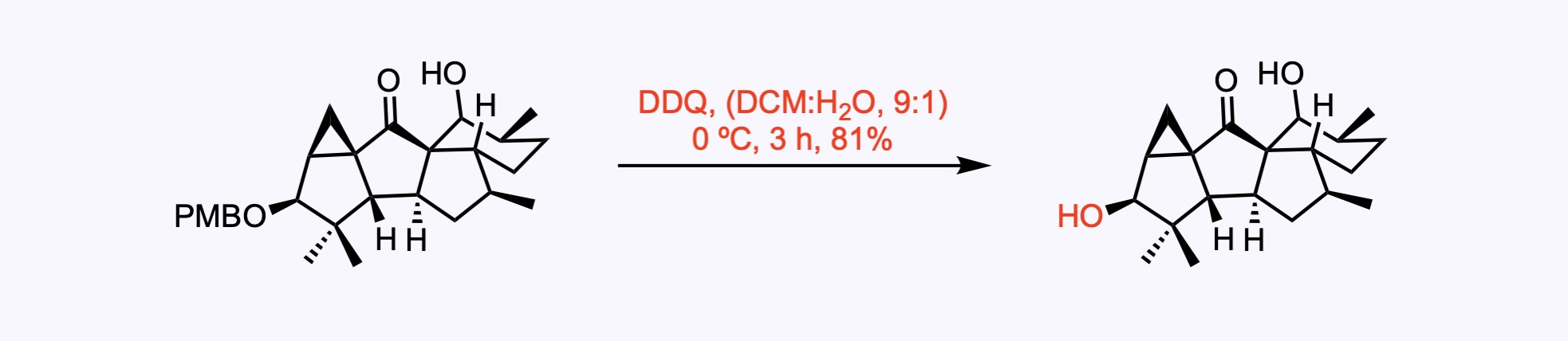
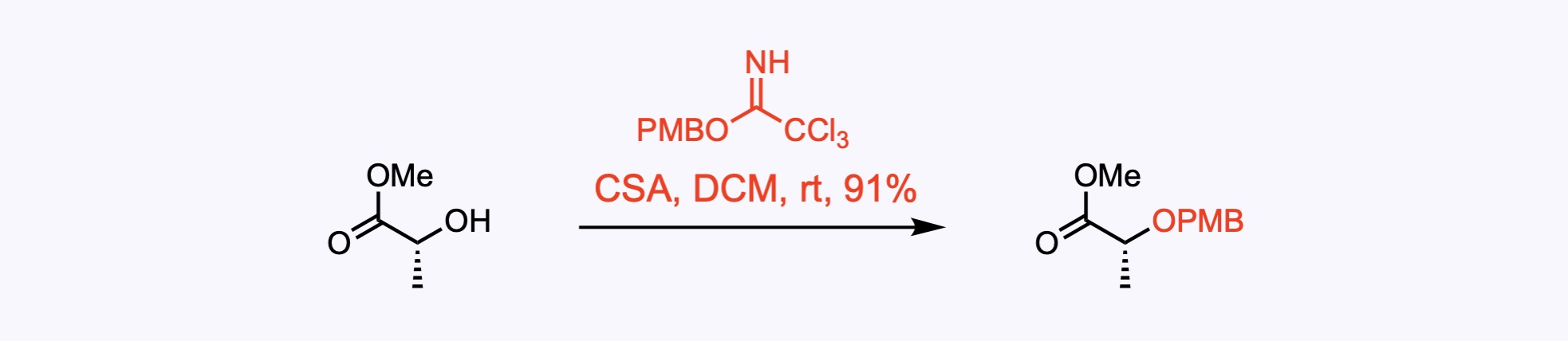
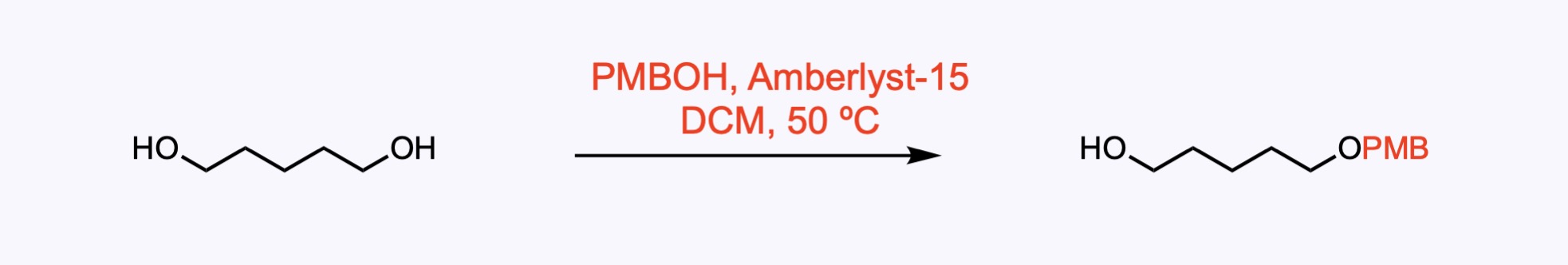
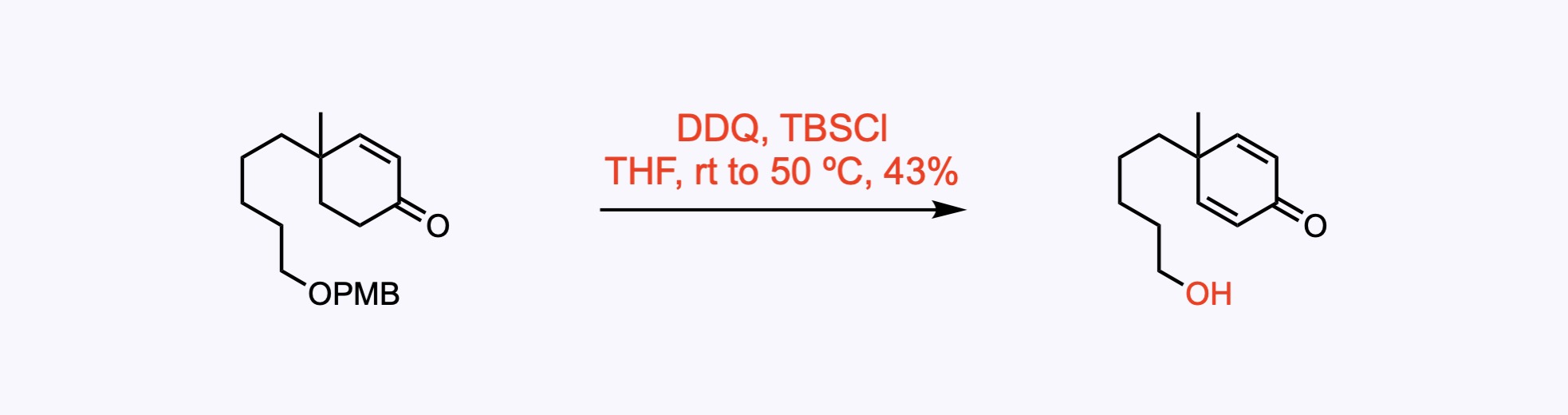
p-Methoxybenzyl ether (PMB) – Removed by acid, hydrogenolysis, or oxidation – commonly with DDQ .
4. For the synthesis of Incargranine A.
3. For the synthesis of Aberrarone.
2. For the synthesis of Amycolamicin.
1. For the synthesis of Adociaquinone A.
Chem. Sci. 2021, 12, 4747. Open access.
TBDPS
tert-Butyldiphenylsilyl, also known as TBDPS; Its formula is C16H19Si-. The increased stability towards acidic hydrolysis and nucleophilic species allows for the TBDPS groups in a substrate to be retained while other silyl ethers are removed. The TMS group may easily be removed in the presence of a TBDPS group by reaction with TsOH.
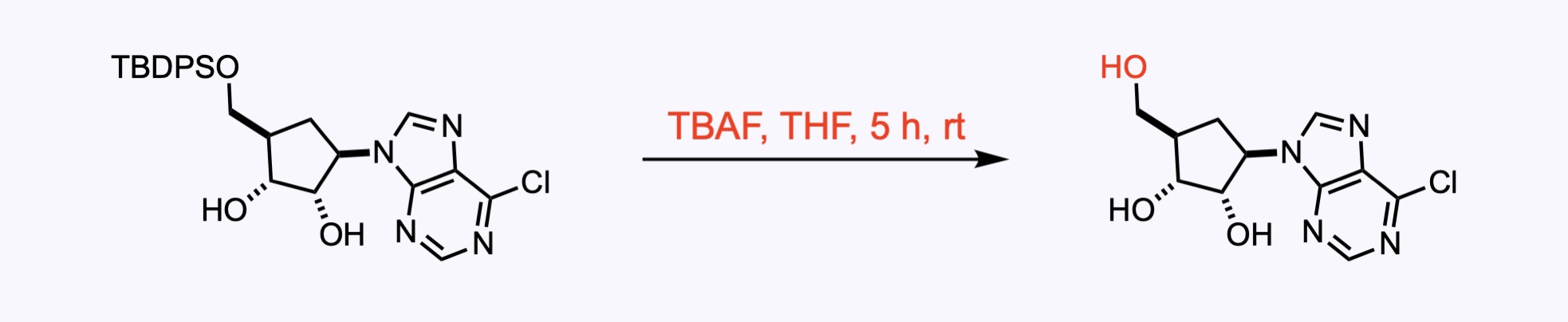
The group is even more resistant to acid hydrolysis than the bulky TIPS. However, in the presence of a fluoride source such as TBAF or TASF, TIPS groups are more stable than TBDPS groups.
It is possible to remove the TBDPS group selectively, leaving a TBDMS group intact, using NaH in HMPA at 0 °C for five minutes.
2. For the synthesis of Aristeromycin.
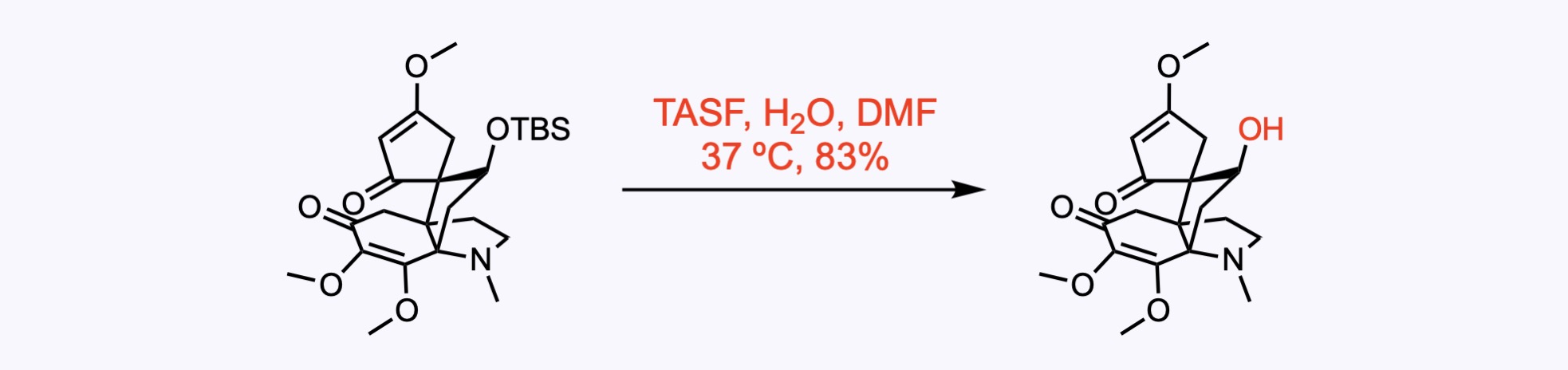
1. For the synthesis of Aberrarone.
TBS
tert-Butyldimethylsilyl, also known as TBS or TBDMS. These silyl ethers hydrolyze much more slowly than the trimethylsilyl ethers.
3. For the synthesis of 10-hydroxyacutuminine.
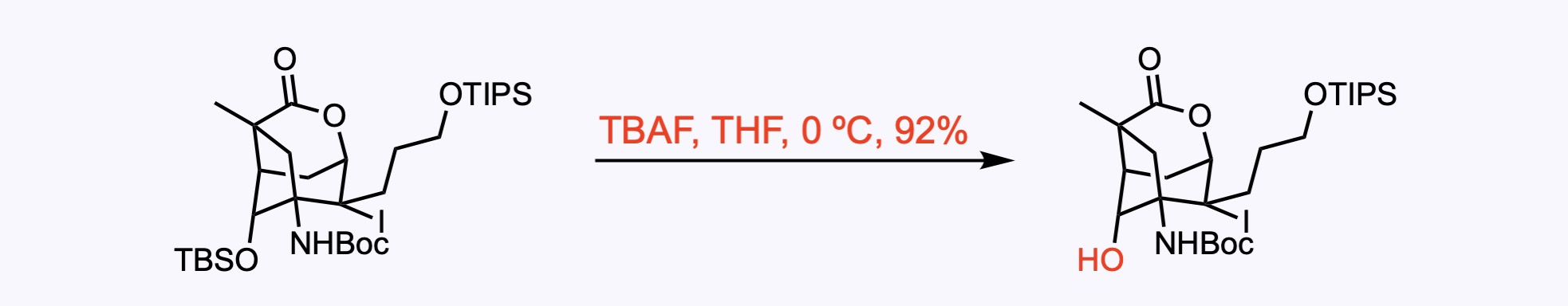
2. For the synthesis of Annotinolide C.
J. Am. Chem. Soc. 2021, 143, 11951. Open access.
1. For the synthesis of Codonopiloneolignanin A.
TES
Triethylsilyl ether.
1. For the synthesis of Norzoanthamine.
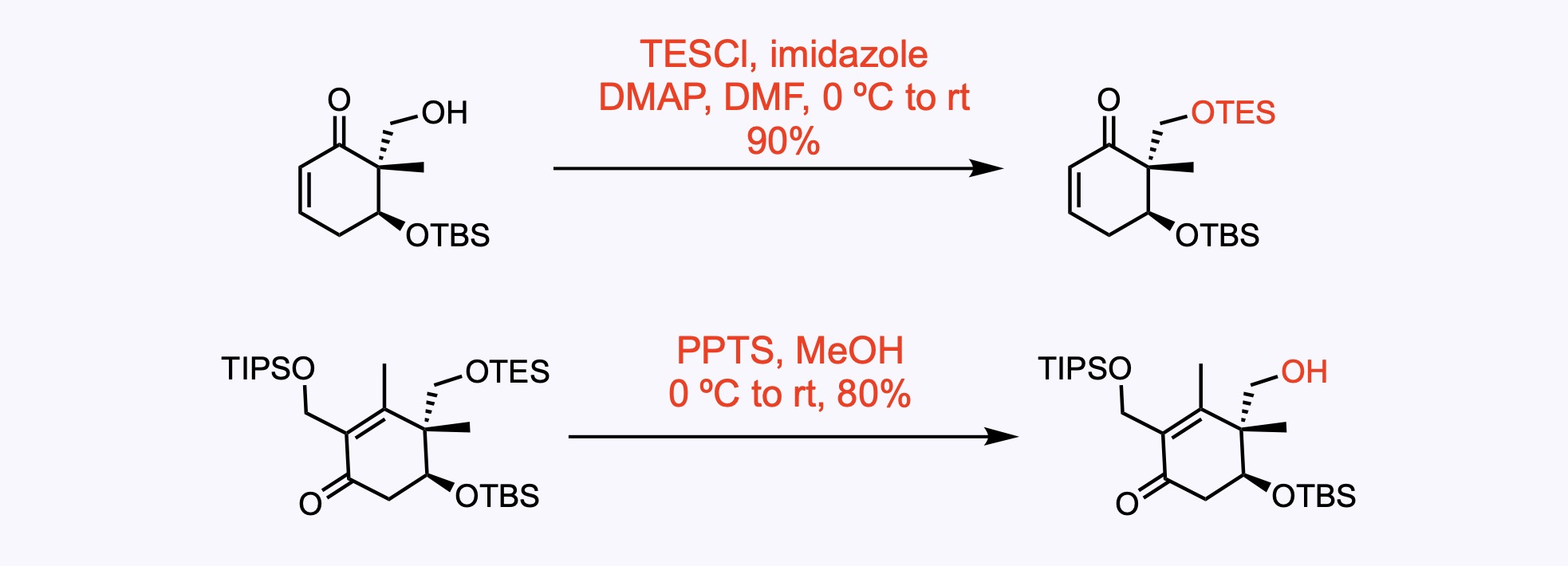
The primary hydroxyl group was protected as a triethylsilyl ether group (-OTES). The TES group was then selectively removed with pyridinium p-toluenesulfonate (PPTS).
TIPS
Triisopropylsilyl ether.
In acidic media, the relative resistance is the following: TMS < TES < TBS < TIPS < TBDPS
In basic media, the relative resistance is the following: TMS < TES < TBS~TBDPS < TIPS
1. For the synthesis of Annotinolide C.
J. Am. Chem. Soc. 2021, 143, 11951. Open access.






0 Comments