Oxidation Reactions
This post is about oxidation reactions in organic chemistry. These are reactions that change the oxidation state of the atoms on the substrate. Thus, oxidation occurs when the oxidation number of an atom becomes larger. The scope of this post is limited to the oxidation of the carbon atom. The post is active and growing, so you can expect more examples to be added over time. Please be sure to come back. The updates will be announced on my instagram account.
Last update 23/08/27.
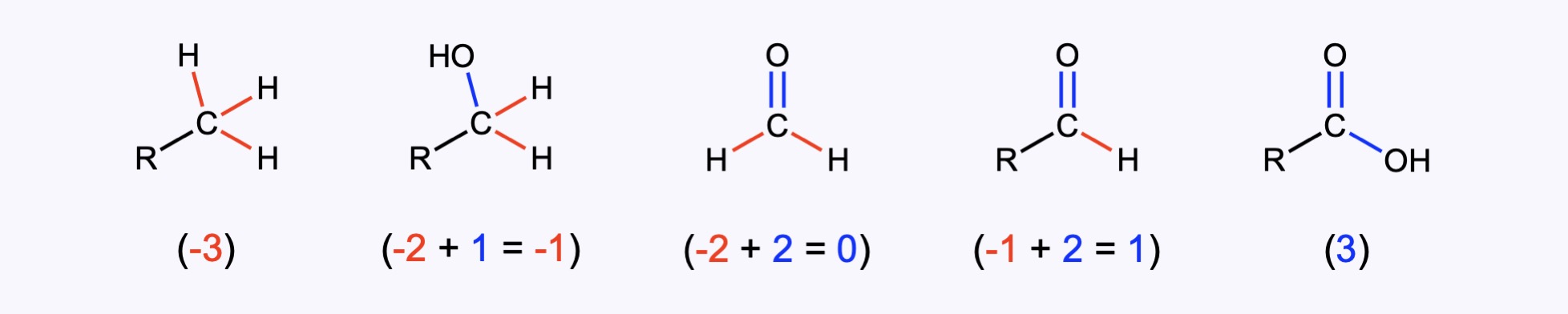
In forming chemical bonds, atoms donate, acquire, or share electrons from their valence shell. Knowing their behavior, makes it possible to assign every atom an oxidation number. The oxidation number specifies the number of electrons of a given atom that can be involved in forming bonds with other atoms. From the particular atoms in a molecule and their known bonding capacities, the bonding pattern within a molecule is determined, and each atom is regarded as being in a specific oxidation state, expressed by an oxidation number.
Unlike metals, which are almost always in a positive oxidation state, the oxidation state of carbon can vary from -4 (in CH4) to +4 (in CO2 or CCl4). By definition, the oxidation state of an atom is the charge that atom would carry if the compound were purely ionic. Even though the charge on the carbon is not really +4 or –4, the oxidation state formalism helps us keep track of where the electrons are going. For example, in a C–H bond, the H is treated as if it has an oxidation state of +1. This means that every C–H bond will decrease the oxidation state of carbon by 1. In contrast, in a C–Cl bond, the Cl is treated as if it has an oxidation state of -1; every C–Cl bond will increase the oxidation state of carbon by 1. Therefore, if a primary alcohol, RCH2OH, is oxidized to the corresponding aldehyde, RCHO, the oxidation state of the C goes from (-2+1=-1) to (-1+2=+1).
As a rule of thumb, every bond between C and C does not affect the oxidation state. Every bond between C and H will decrease the oxidation state by 1. And every single bond between C and a more electronegative element (O, N, Cl) will increase its oxidation state by 1.
Alpha-Hydroxylation
Oxidation reactions where the alpha position of a carbonyl compound gets hydroxylated.
The alpha hydroxylation of a ketone is a chemical reaction that involves the addition of a hydroxyl group to the carbon atom adjacent to a carbonyl group. This process is often used in organic synthesis to create a variety of useful compounds and to add functionality onto the molecule. The reaction typically involves the use of an oxidizing agent, such as hydrogen peroxide, to add the hydroxyl group to the ketone. The resulting product will vary depending on the specific conditions used.
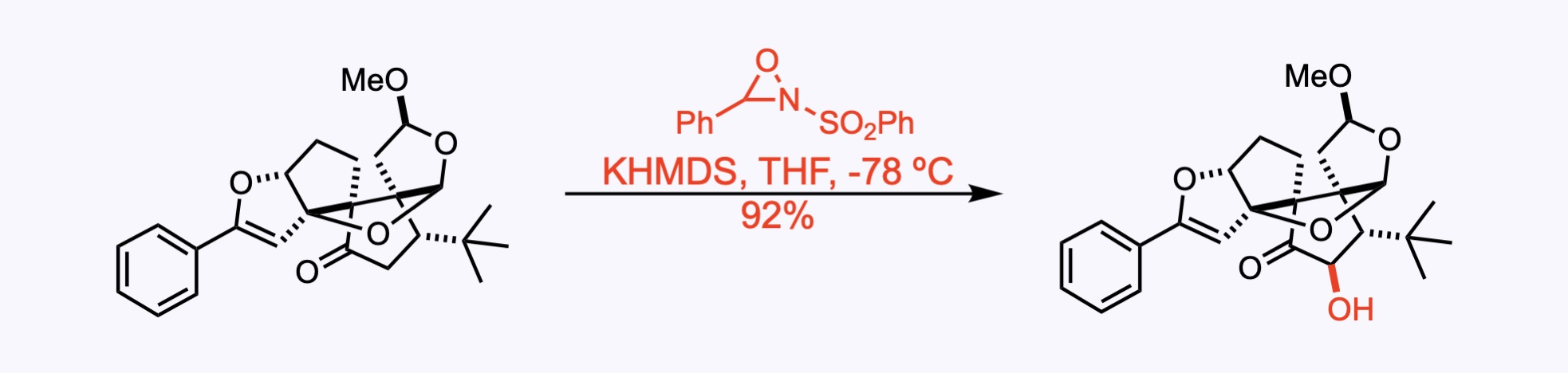
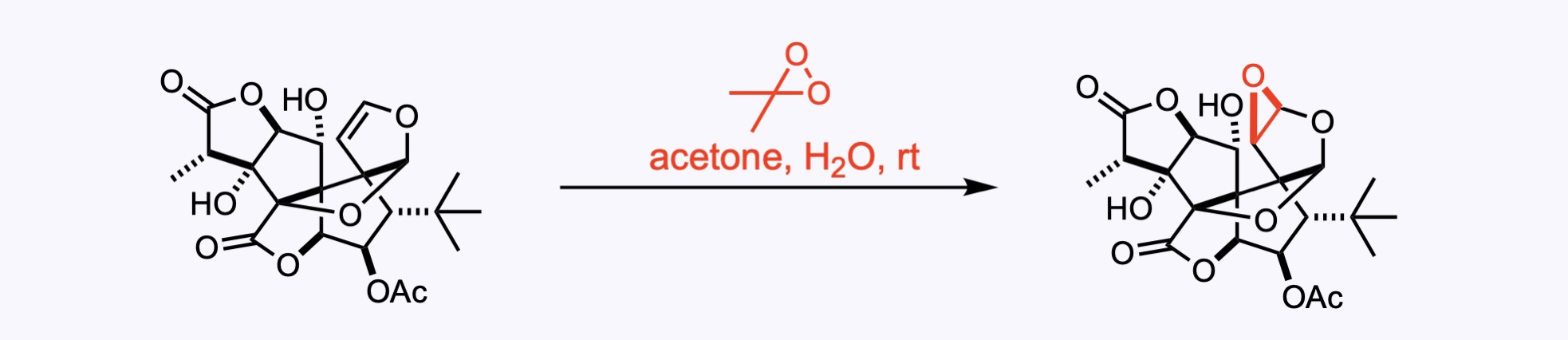
3. For the synthesis of Ginkgolide C.
An α-hydroxylation with the Davis’ oxaziridine.
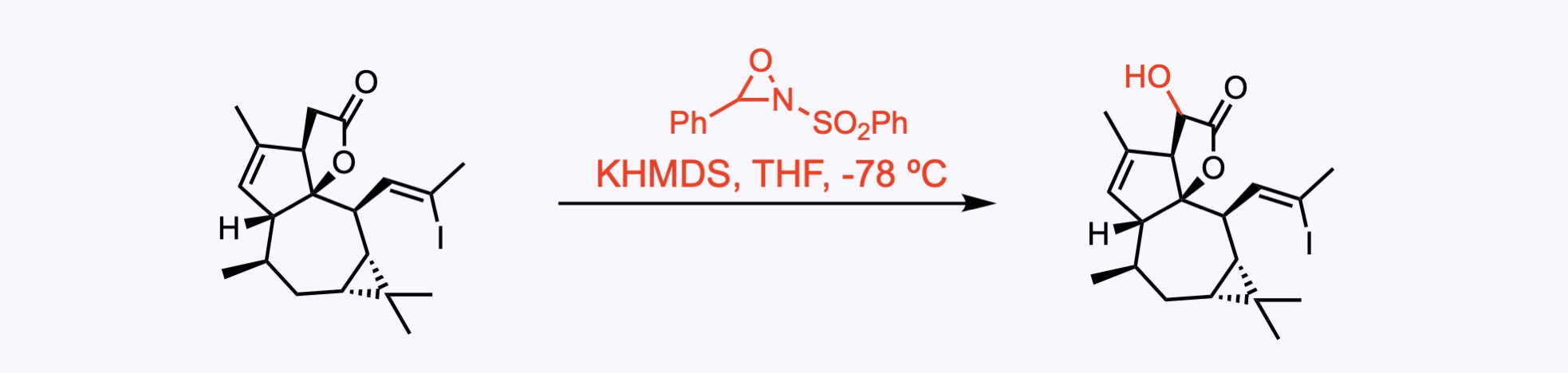
2. For the synthesis of Euphorikanin A.
An α-hydroxylation with the Davis’ oxaziridine. The use of 2-(phenylsulfonyl)-3-phenyloxaziridine (Davis reagent) or similar oxaziridine reagents allows the oxidation of enolates generated in situ from ketones or esters to provide α-hydroxylated compounds.
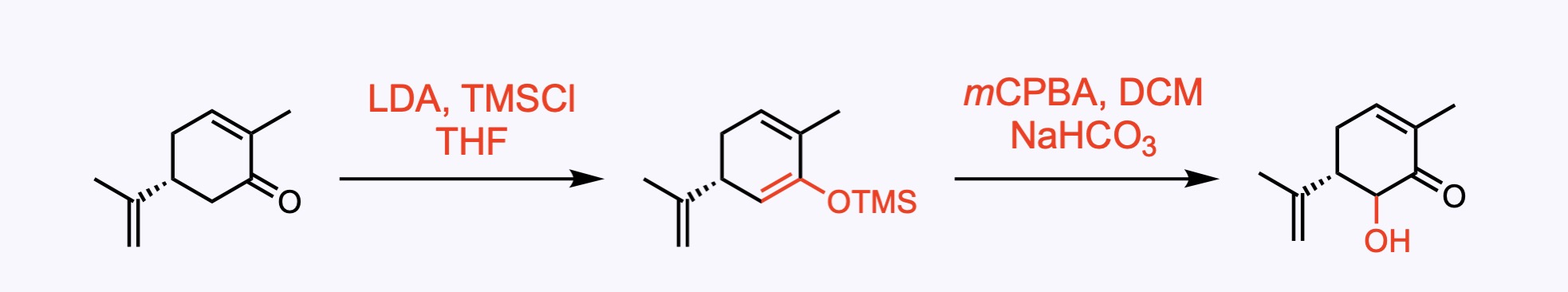
1. For the synthesis of Euonyminol.
Rubottom oxidation: alpha-hydroxylation of a ketone by the chemical reaction between silyl enol ethers and peroxyacids.
Enone Formation
Organic reactions where a ketone is oxidized to the corresponding alpha,beta-unsaturted ketone. In some cases, the enone is the end product from the oxidation of another substrate.
6. For the synthesis of Asnovolin A.
IBX was identified as an optimal oxidant to effect desaturation of the ketone to produce the enone with complete regiocontrol in good yield (72%). Addition of 4 Å molecular sieves and Na2HPO4 was crucial to prevent acetal hydrolysis.
5. For the synthesis of Undulifoline.
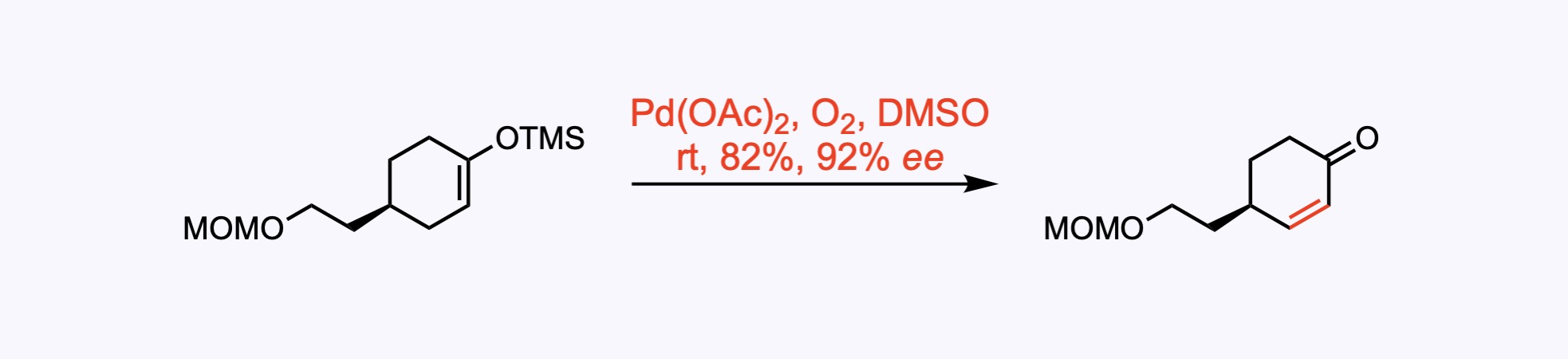
Saegusa oxidation, a chemical reaction used in organic chemistry as a method to introduce α-β unsaturation in carbonyl compounds. The reaction involves formation of a silyl enol ether followed by treatment with palladium(II) acetate and benzoquinone (oxygen gas in this case) to yield the corresponding enone.
4. For the synthesis of Undulifoline.
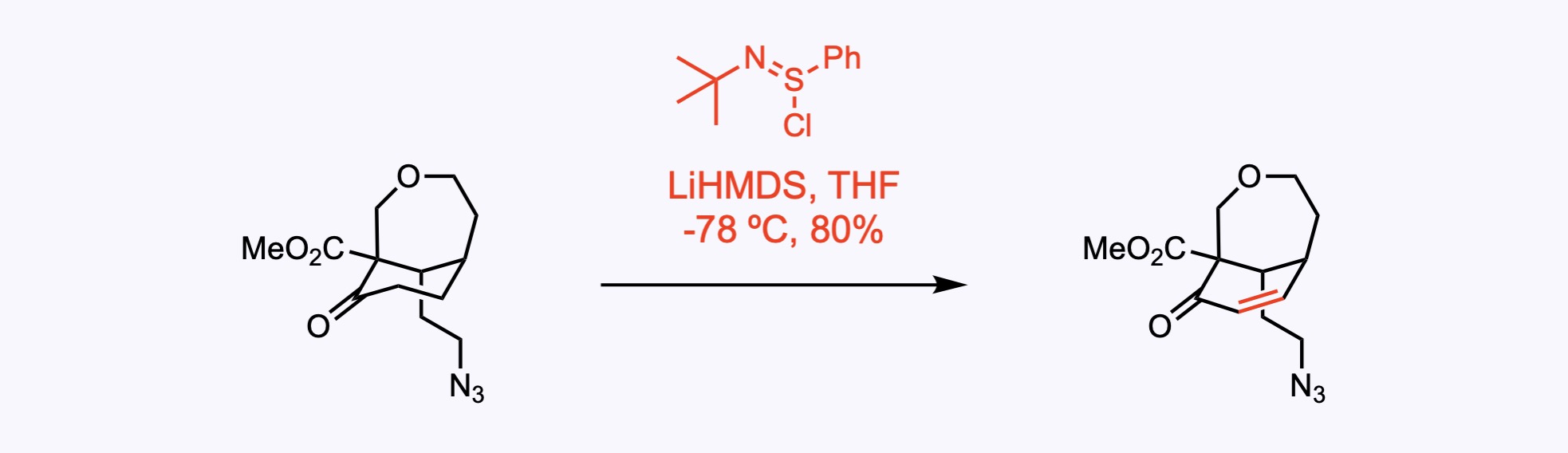
The α,β-unsaturated ketone was synthesized by a one-pot procedure from the starting ketone in 80% yield on treatment of its lithium enolate salt with N-tert-butyl phenylsulfinimidoyl chloride under mild reaction conditions. Further references regarding this innovative procedure: Chem. Lett. 2000, 29, 1250.
3. For the synthesis of Phomarol.
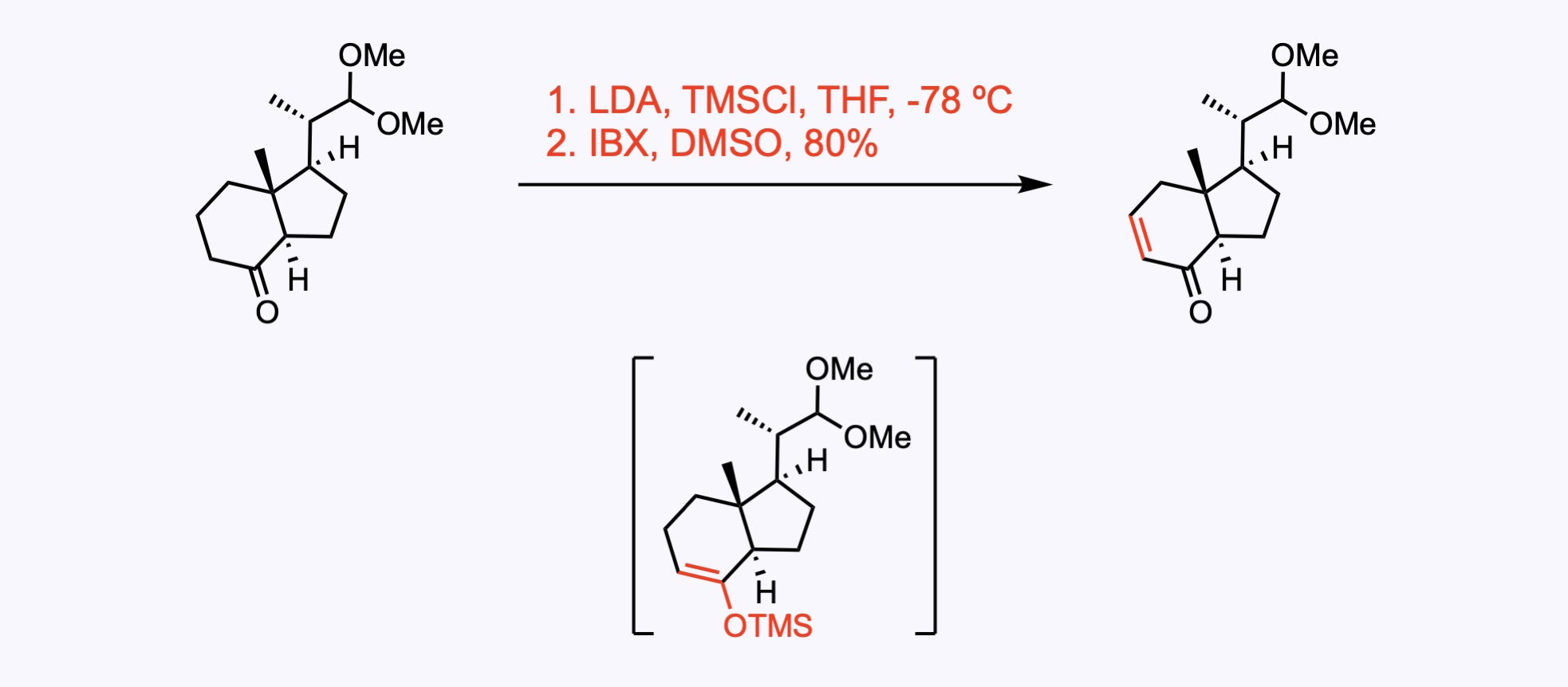
Formation of the silyl enol ether followed by oxidation with 2-iodoxybenzoic acid, IBX.
CCS Chem. 2021, 3, 348. Open access.
2. For the synthesis of Norzoanthamine.
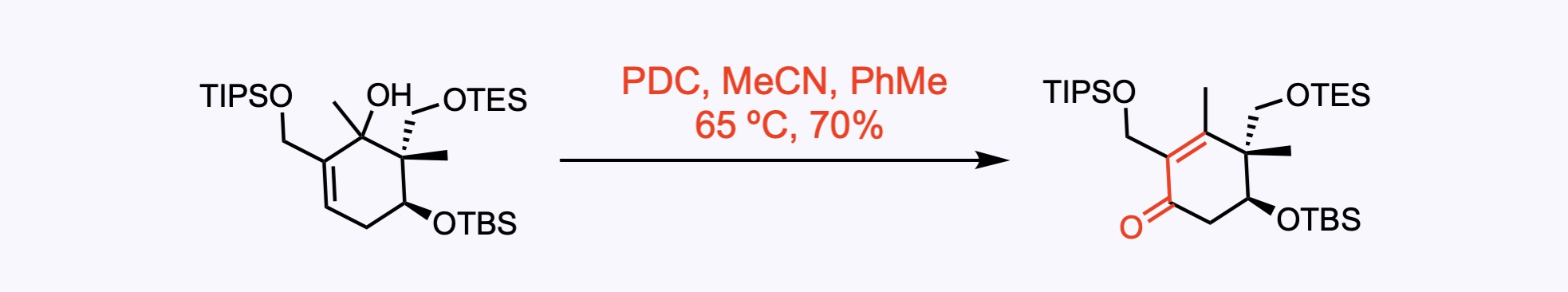
Babler-Dauben oxidation. Pyridinium chlorochromate (PCC) was first used as the oxidant, but it led to the decomposition of the acid-sensitive substrate. Therefore, the milder oxidant pyridinium dichromate (PDC) was applied at 65 ºC and afforded the desired penta-substituted cyclohexanone in 70% yield on decagram scale.
1. For the synthesis of Cephanolide A.
Riley oxidation: The selenium dioxide-mediated oxidation of olefins at the allylic position. This transformation allows the formation of the allylic alcohol that is later oxidized to the corresponding enone by the use of Dess–Martin periodinane.
Epoxidation Reactions
Oxidation of alkenes to yield the corresponding epoxide products.
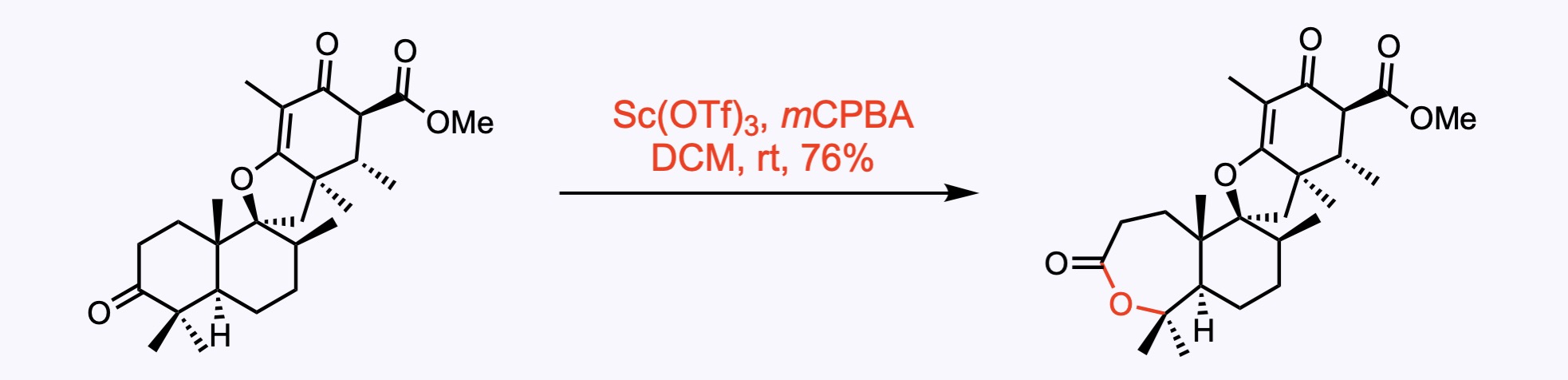
7. For the synthesis of Ginkgolide C.
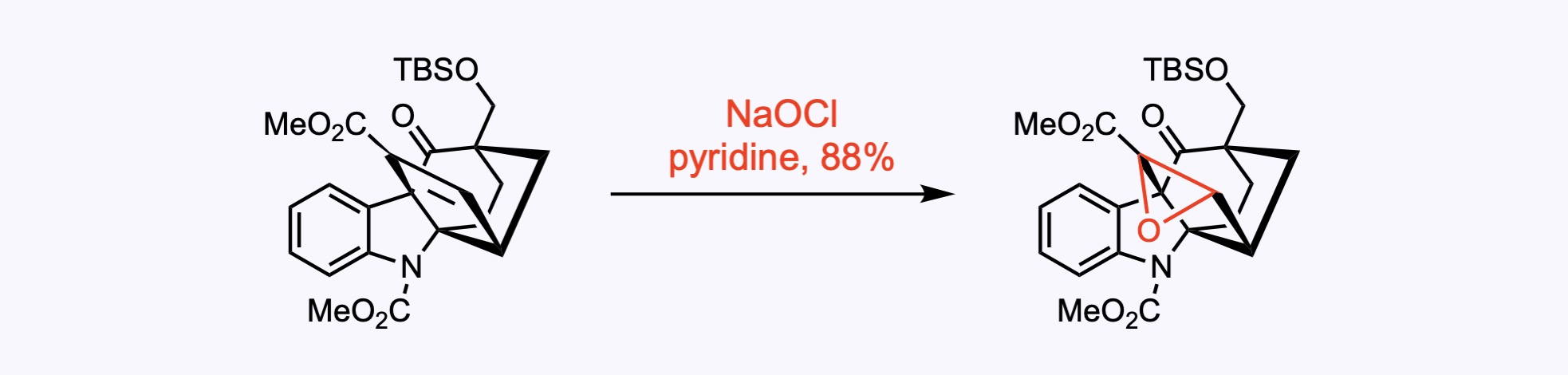
6. For the synthesis of 10,22-Dioxokopsane.
For this transformation, sodium hypochlorite (14.5 wt.% aqueous solution, 3.0 equiv.) was used as a good source of oxygen for olefin epoxidation under mild reaction conditions. The reaction was carried out in pyridine at 0 ºC for 30 min, yielding the desired product in 88% yield.
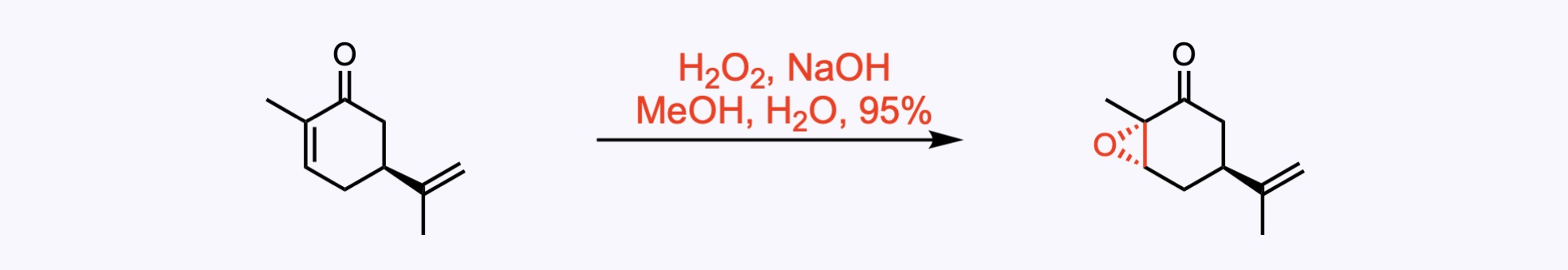
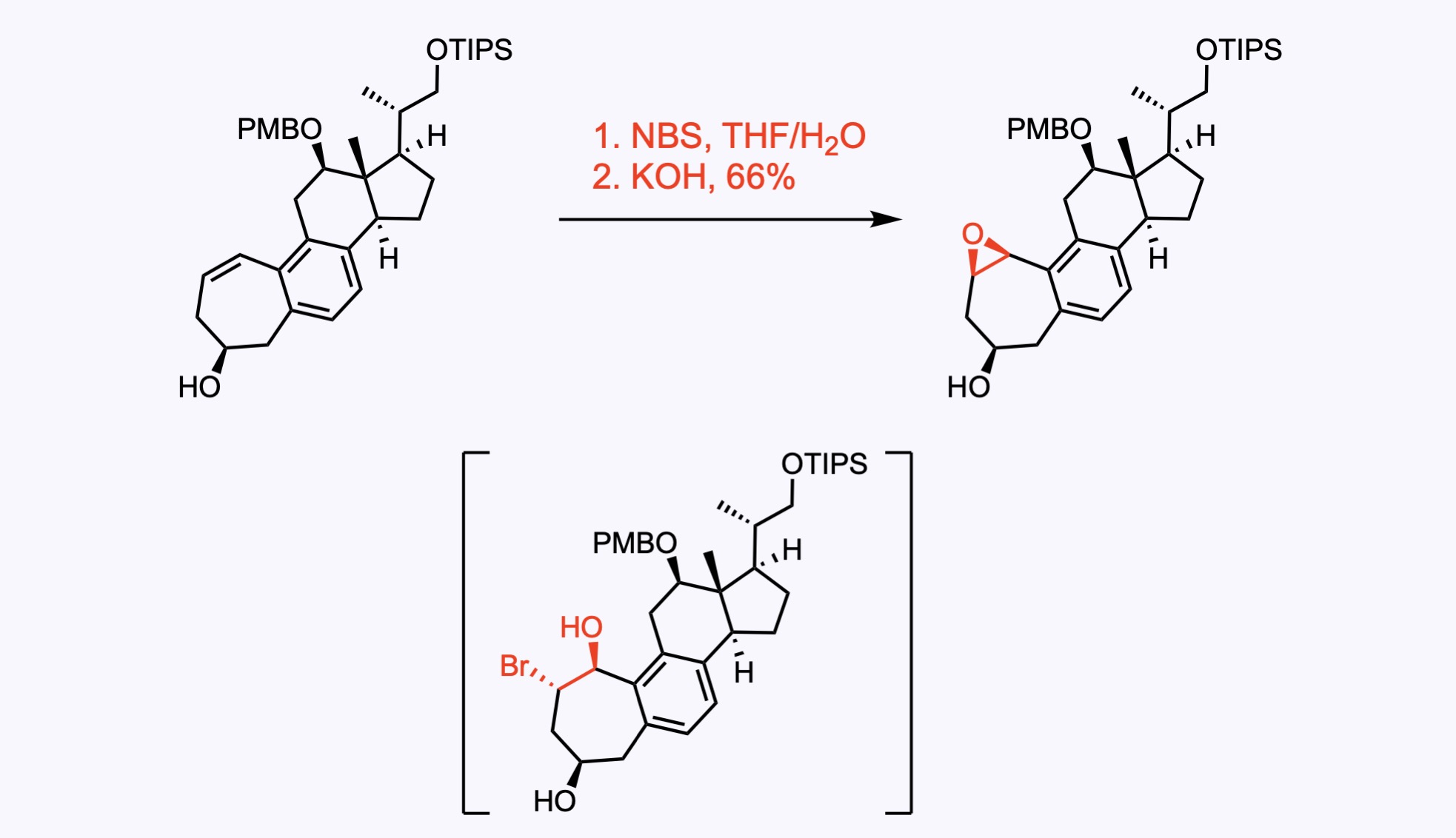
5. For the synthesis of Ambiguine G.
J. Am. Chem. Soc. 2021, 143, 10872. Open access.
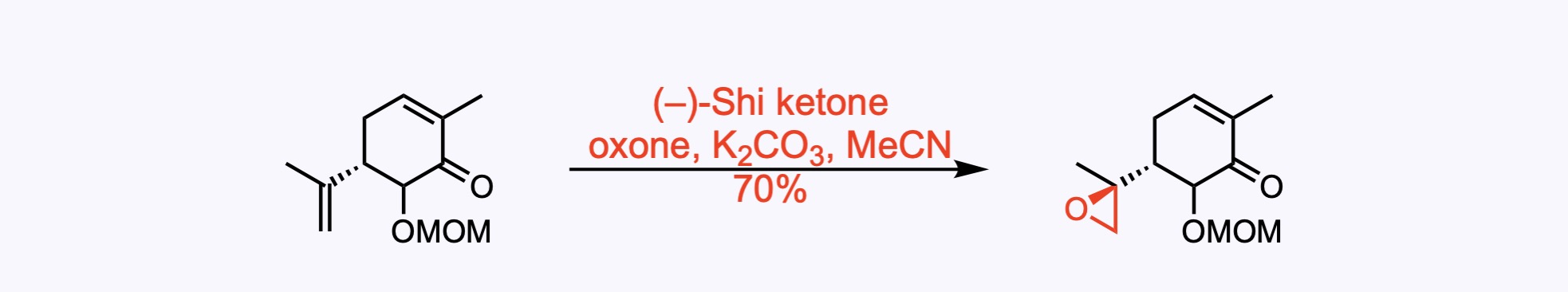
4. For the synthesis of Euonyminol.
Shi asymmetric epoxidation. For the epoxidation of alkenes with oxone (potassium peroxymonosulfate) and a fructose-derived catalyst, the Shi ketone.
3. For the synthesis of Phomarol.
Bromohydroxylation of the double bond, followed by cyclization using KOH as a base gave the desired epoxide.
CCS Chem. 2021, 3, 348. Open access.
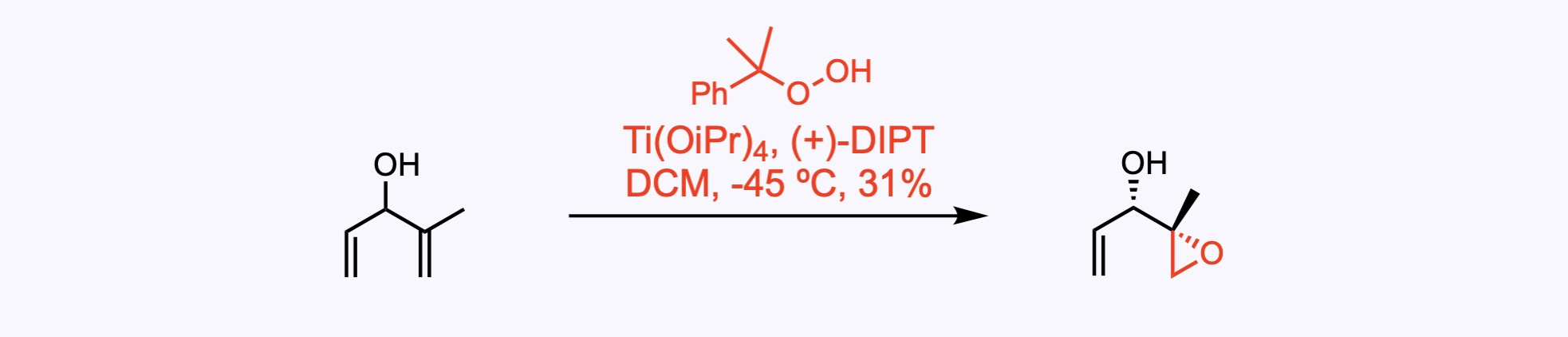
2. For the synthesis of Pladienolide B.
Sharpless asymmetric epoxidation. The product was obtained in 31% yield, 95% ee, and >20:1 dr.
1. For the synthesis of Lefamulin.
Exposure of the olefin to mCPBA resulted in syn epoxidation of the carbamate.
Oxidation of Alcohols
Oxidation of alcohols to the corresponding aldehydes, ketones or carboxylic acids.
10. For the synthesis of Longiborneol.
9. For the synthesis of Alstonlarsine A.
Corey-Kim oxidation: for the synthesis of aldehydes and ketones from primary and secondary alcohols using N-chlorosuccinimide (NCS), dimethyl sulfide (DMS), and an organic base (TEA).
8. For the synthesis of Alstonlarsine A.
7. For the synthesis of Aberrarone.
Parikh-Doering oxidation: The oxidation that uses dimethyl sulfoxide as the oxidant, activated by sulfur trioxide pyridine complex in the presence of a base such as DIPEA or triethylamine.
6. For the synthesis of Euphorikanin A.
5. For the synthesis of Thebainone A.
4. For the synthesis of Cyclobutastellettolide B.
Swern oxidation: The oxidation of alcohols by DMSO and oxalyl chloride.
3. For the synthesis of Codonopiloneolignanin A.
Ley-Griffith oxidation: The tetra propylammonium perruthenate (TPAP) catalyzed oxidation of primary or secondary alcohols to aldehydes or ketones. The co-oxidant N-methylmorpholine N-oxide (NMO) is used in stoichiometric amounts to reoxidize the ruthenium catalyst (RuV -> RuVII) and mantain the catalytic cycle.
2. For the synthesis of Annotinolide C.
Oxidation of the secondary alcohol to the ketone with Dess–Martin periodinane, DMP.
J. Am. Chem. Soc. 2021, 143, 11951. Open access.
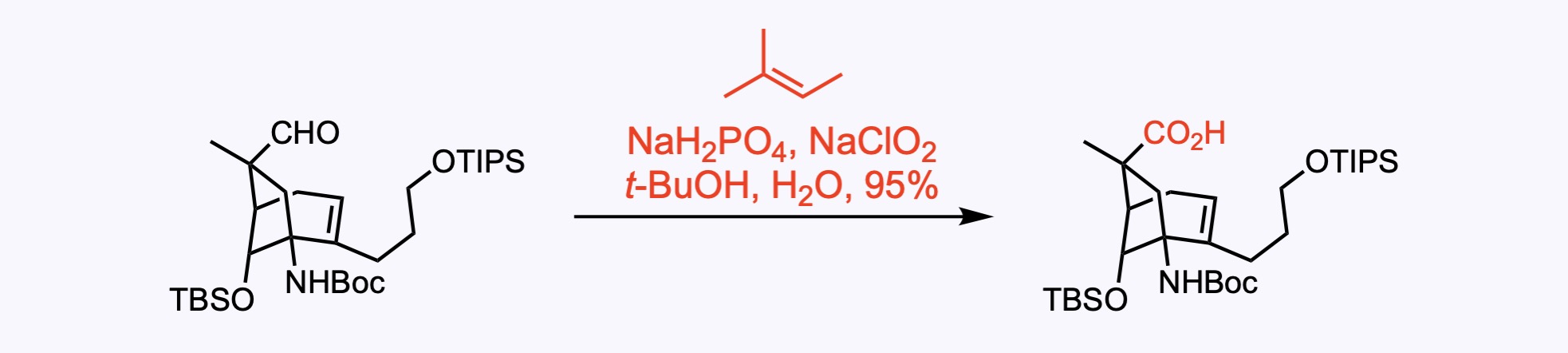
1. For the synthesis of Lemborexant.
A one-pot two step oxidation of the primary alcohol to the carboxylic acid facilitated by TEMPO and NaOCl, followed by treatment with NaClO2 and pH adjustment.
Oxidation of Ketones
2. For the synthesis of Asnovolin A.
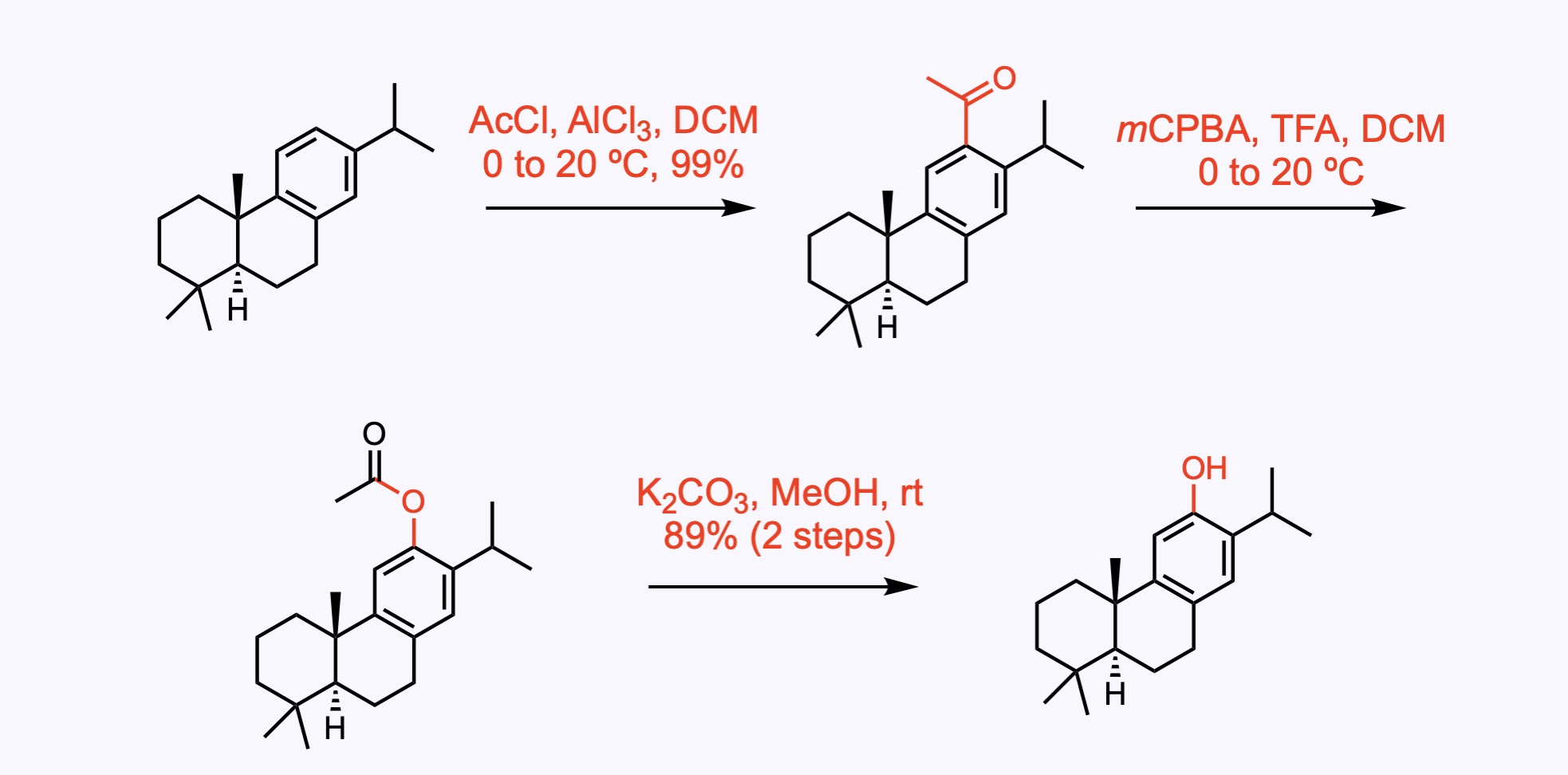
1. For the synthesis of Maytenone.
Regioselective Friedel–Crafts acetylation and a Baeyer–Villiger oxygenation.
Oxidation Chemical Reactions
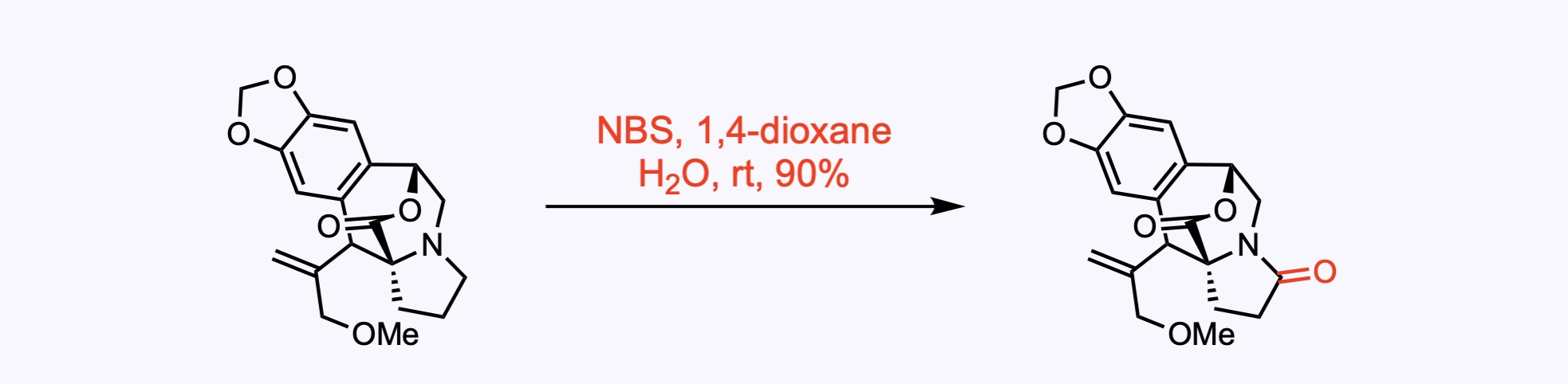
2. For the synthesis of Drupacine.
The tertiary amine was oxidized to amide using NBS.
1. For the synthesis of Annotinolide C.
J. Am. Chem. Soc. 2021, 143, 11951. Open access.















0 Comments