Overman Rearrangement
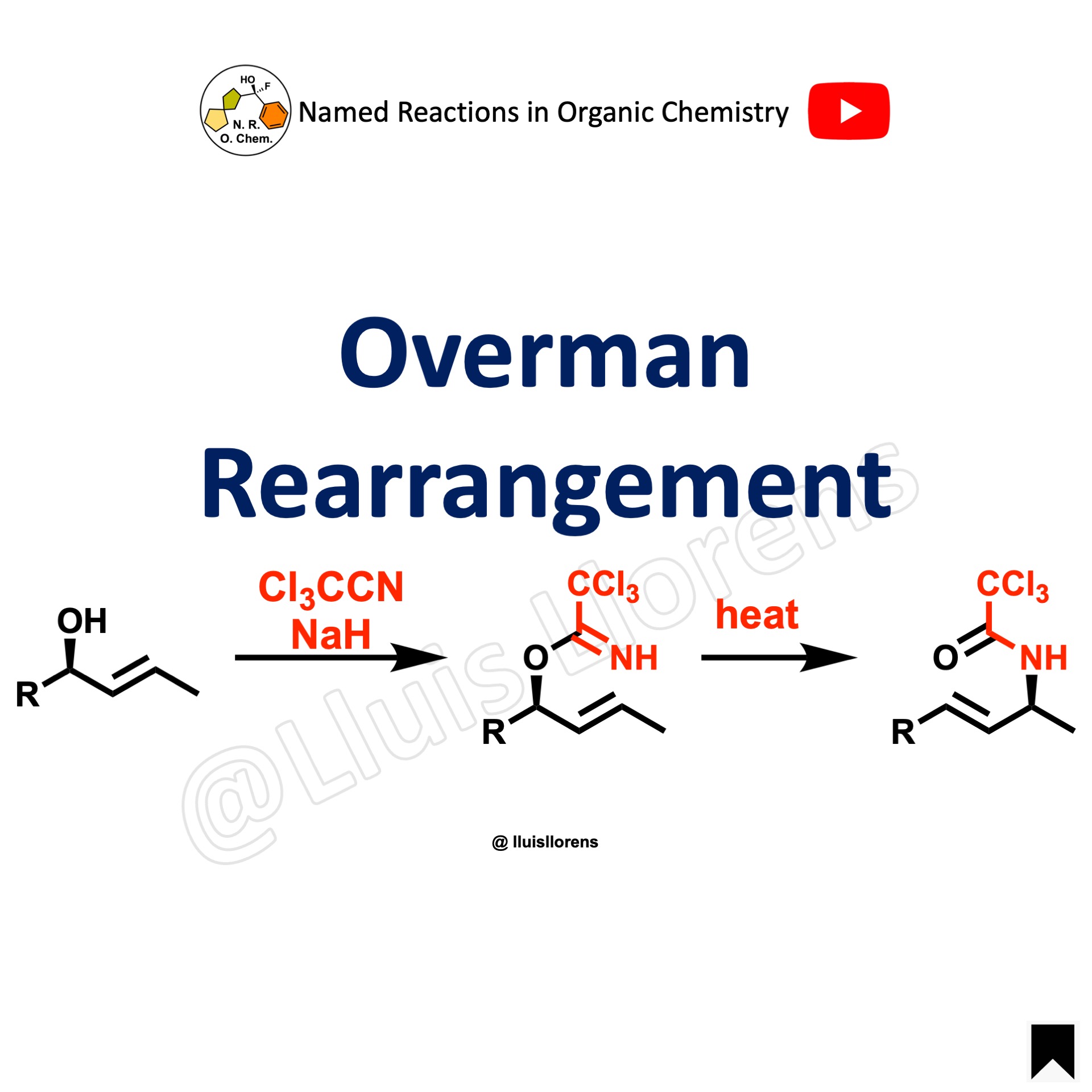
The Overman rearrangement is the diastereoselective [3,3]-sigmatropic rearrangement of allylic alcohols to give allylic trichloroacetamides through an imidate intermediate.
Similar to the mechanism of the Claisen rearrangement, the Overman rearrangement is a suprafacial, concerted rearrangement that proceeds through a six-membered chairlike transition state. Moreover, the formation of the amide functionality is the driving force that makes this reaction irreversible. Besides, the rearrangement of trichloroacetimidates derived from secondary allylic alcohols proceeds with a high level of stereoselectivity and preferentially the (E)-alkenes are formed. Additionally, the amide can be readily hydrolyzed under basic conditions to the corresponding amine.
Reaction Mechanism
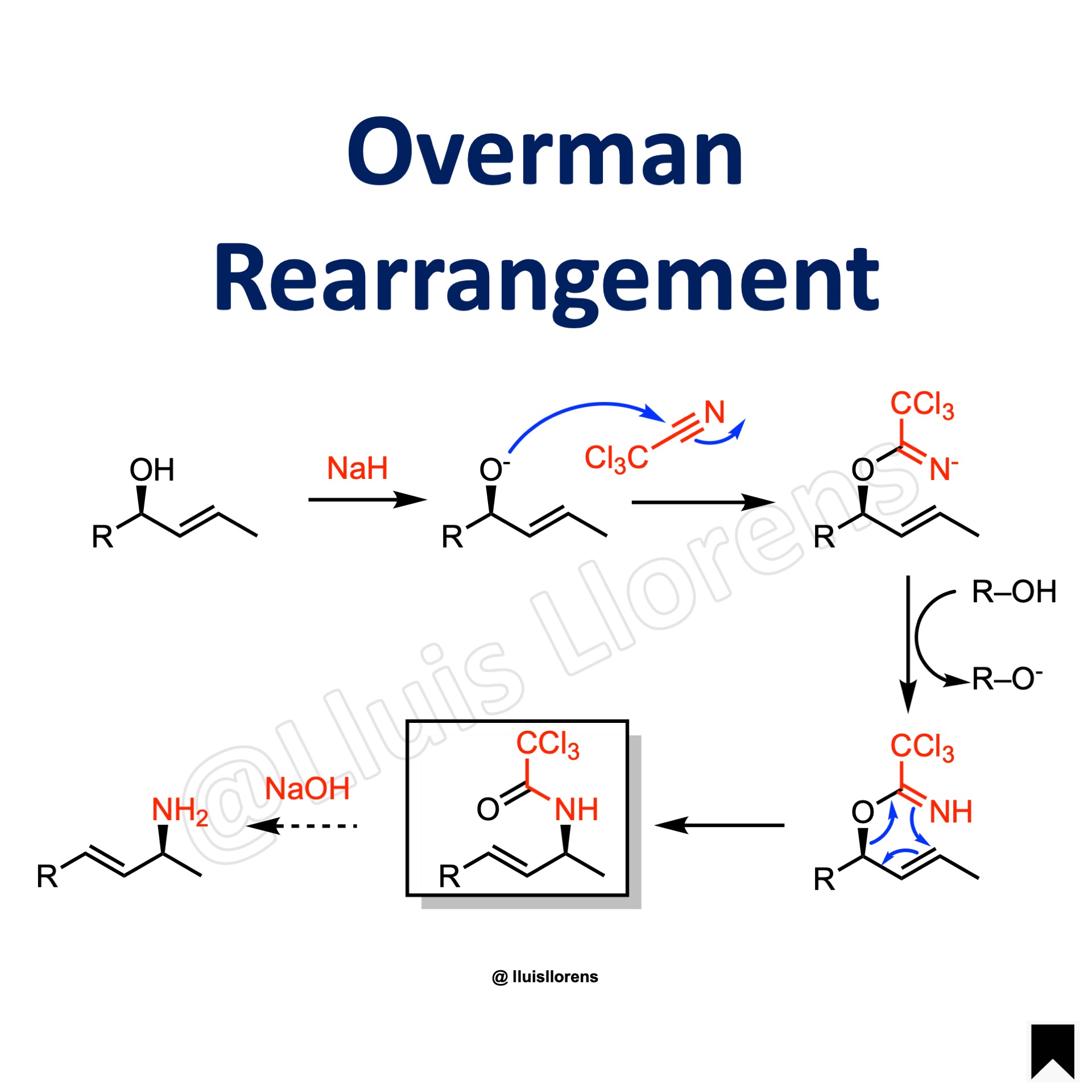
1. The alcohol is first deprotonated by the base. 2. The deprotonated alcohol ads to trichloroacetonitrile to give a trichloroacetimidate anion. 3. The anion abstracts a proton from the alcohol. 4. A thermal [3,3]-sigmatropic rearrangement delivers the allylic trichloroacetamide, which can be readily hydrolyzed under basic conditions to the corresponding amine.
Experimental Procedure
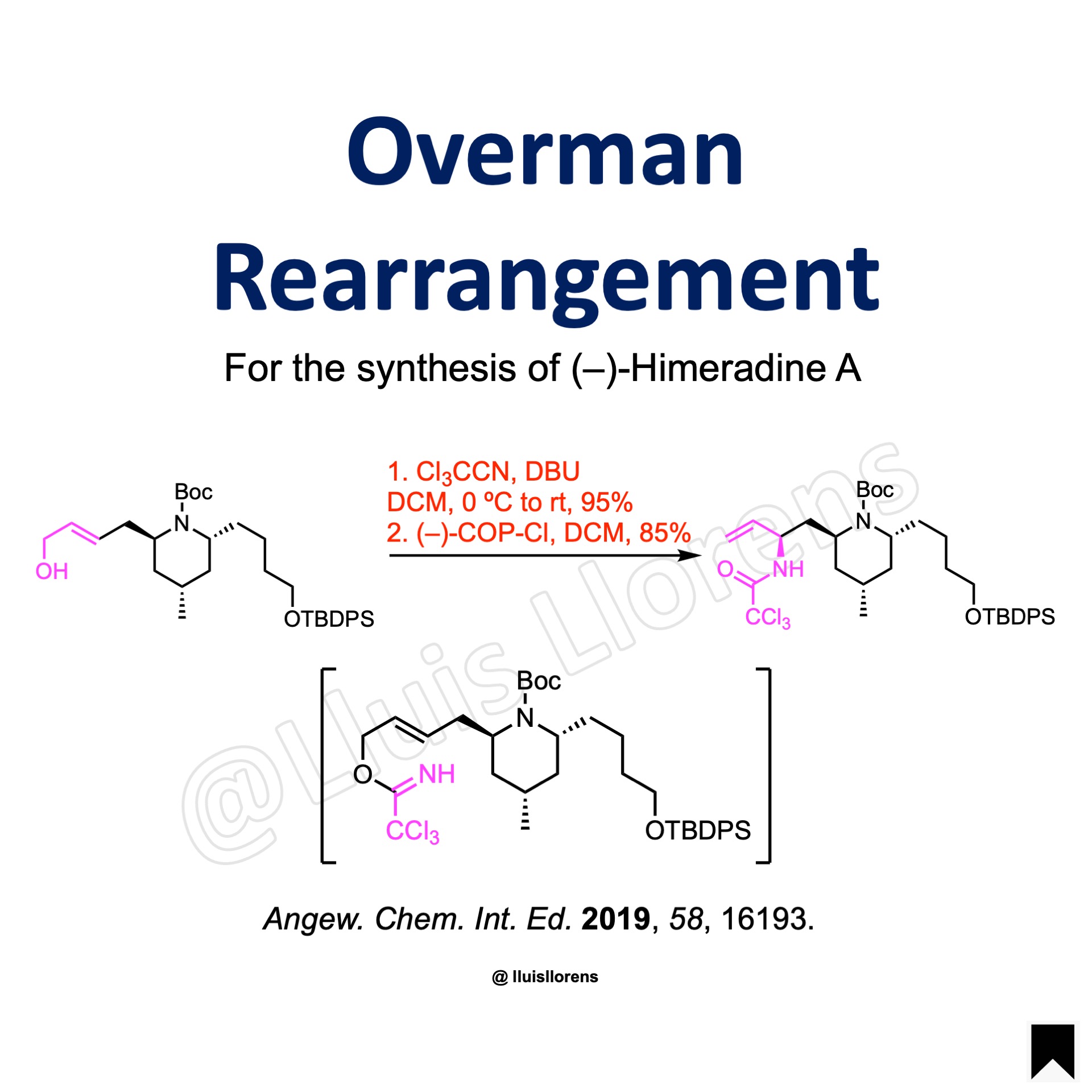
The allylic alcohol (15.8 mmol, 1.0 eq) was dissolved in CH2Cl2 (96 mL), DBU (0.2 eq) was added and the solution cooled to 0 °C. Trichloroacetonitrile (1.5 eq) was added dropwise, and the solution was warmed to room temperature and stirred for 2 h. The solution was concentrated and purified by flash column chromatography on silica gel to deliver the imidate intermediate (95% yield). A flask was charged with the trichloroimidate (14.6 mmol, 1.0 eq), R-(–)-COP-Cl (5.0 mol%), and CH2Cl2 (29 mL) and stirred for 18 h at room temperature. The solution was concentrated and purified by flash column chromatography on silica gel to yield the corresponding trichloroacetamide (85% yield).
Learn More Named Reactions
[instagram-feed feed=2]