Oliceridine
Oliceridine is an opioid medication that was approved by the FDA for the treatment of moderate to severe acute pain in adults. The medication is indicated for short-term intravenous use in hospitals and other controlled clinical settings.
Disease: CNS
Target: μ-opioid receptor (MOR).
Mechanism of Action: G protein-biased μ-opioid receptor agonist.
Opioids are the most effective drugs for the treatment of acute and chronic pain, and they occupy the largest market share of analgesic medications. Medical uses of opioids for pain relief include opioid alkaloids such as morphine and synthetic opioids like fentanyl. Although opioids are potent painkillers, they also result in severe adverse effects such as addiction, respiratory suppression, and constipation, thus limiting their clinical use.
Oliceridine – Mechanism of Action
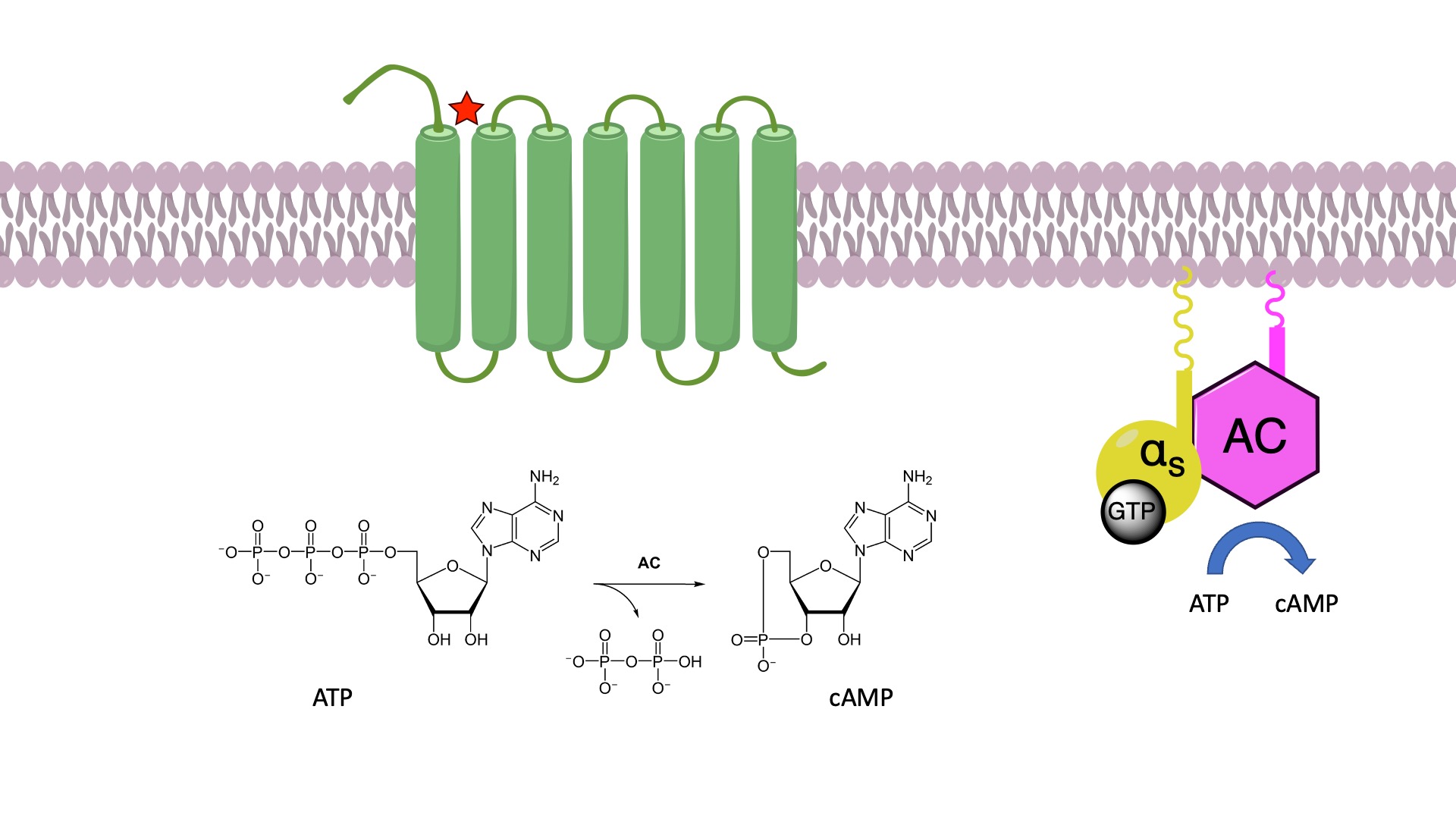
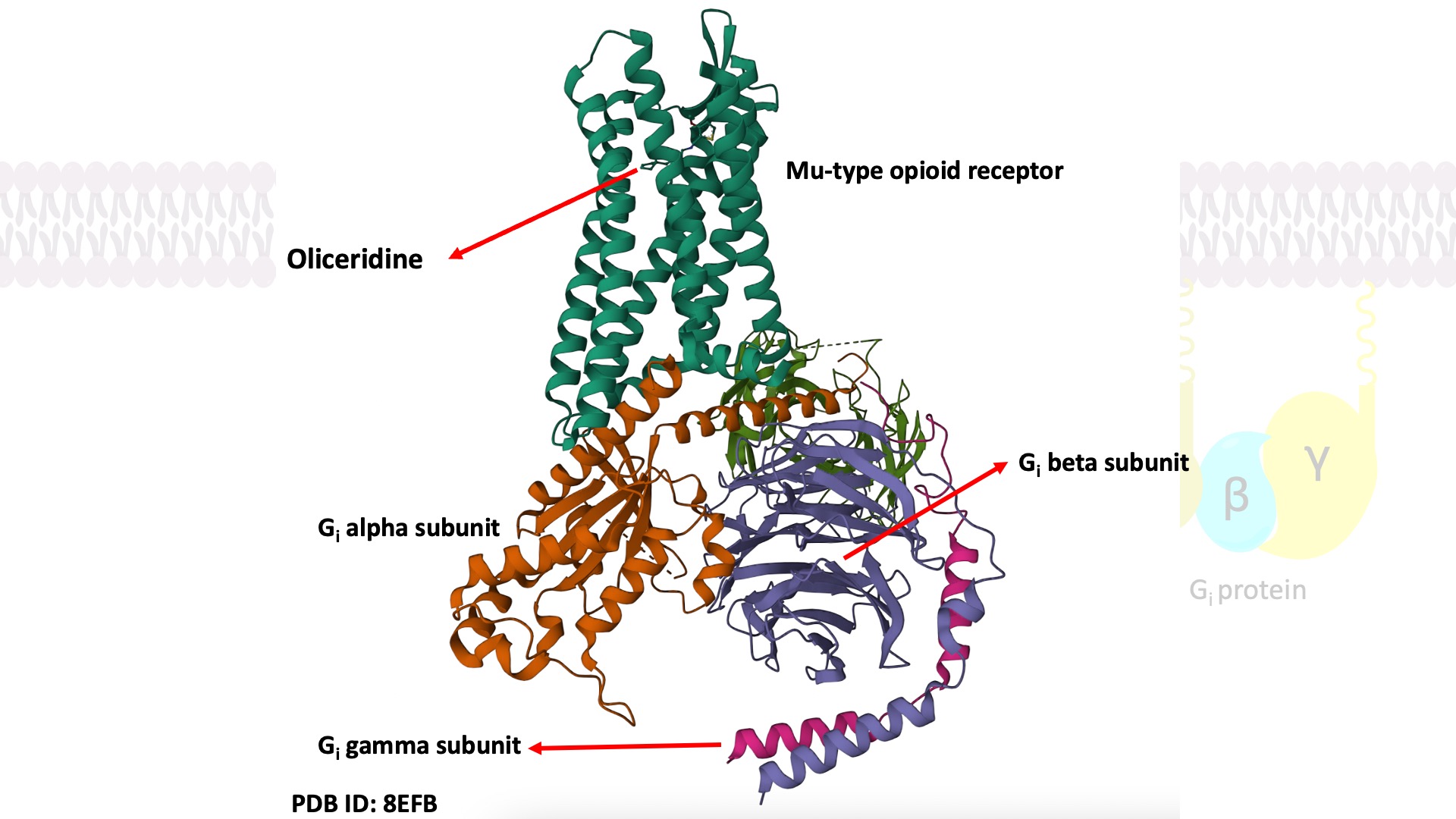
The pharmacological actions of such opioids are mediated by agonism of the μ-opioid receptor (MOR), a G-protein-coupled receptor (GPCR) that is highly expressed in the central nervous system (CNS). GPCRs are seven-transmembrane (7TM) receptors. The receptor protein is embedded within the membrane, with the binding site for the chemical messenger exposed on the outer surface. On the inner surface, there is another binding site which is normally closed. When a chemical messenger binds to its binding site, the receptor protein undergoes a conformational change. This change of shape opens up the binding site on the inner surface. This new binding site is then recognized by a specific G-protein. G-proteins are attached to the inner surface of the cell membrane and are made up of three protein subunits. Upon binding to the receptor, the G-protein changes shape, allowing the release of the GDP located at the alpha subunit. The empty binding pocket has now the right shape to bind GTP. Binding of GTP results in another conformational change, and the subunits dissociate to form a monomer and a dimer. These subunits depart the receptor and initiate a signaling cascade. There is substantial amplification of the signal in this process, because one activated receptor activates several G-proteins before the ligand departs, switching off the receptor.
There are several different G-proteins, which are recognized by different types of receptors. Some of the activated subunits from these G-proteins have an inhibitory effect, while others have a stimulatory effect. For example, the alpha s subunit of the Gs-protein binds to a membrane-bound enzyme called adenylate cyclase. Upon binding, the enzyme catalyzes the synthesis of cyclic AMP also known as cAMP. cAMP is a secondary messenger that moves into the cell’s cytoplasm and carries the signal from the cell membrane into the cell itself.
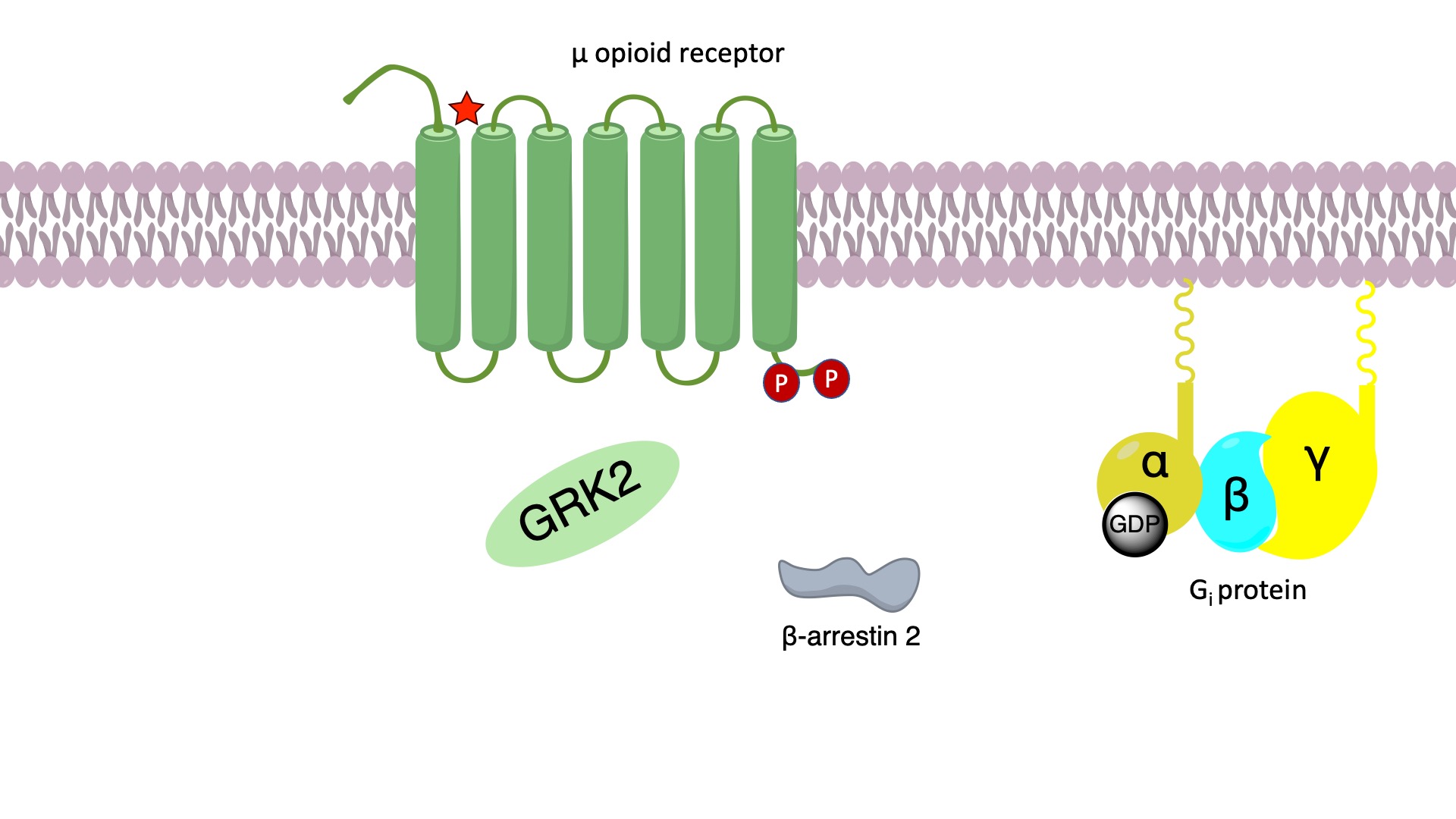
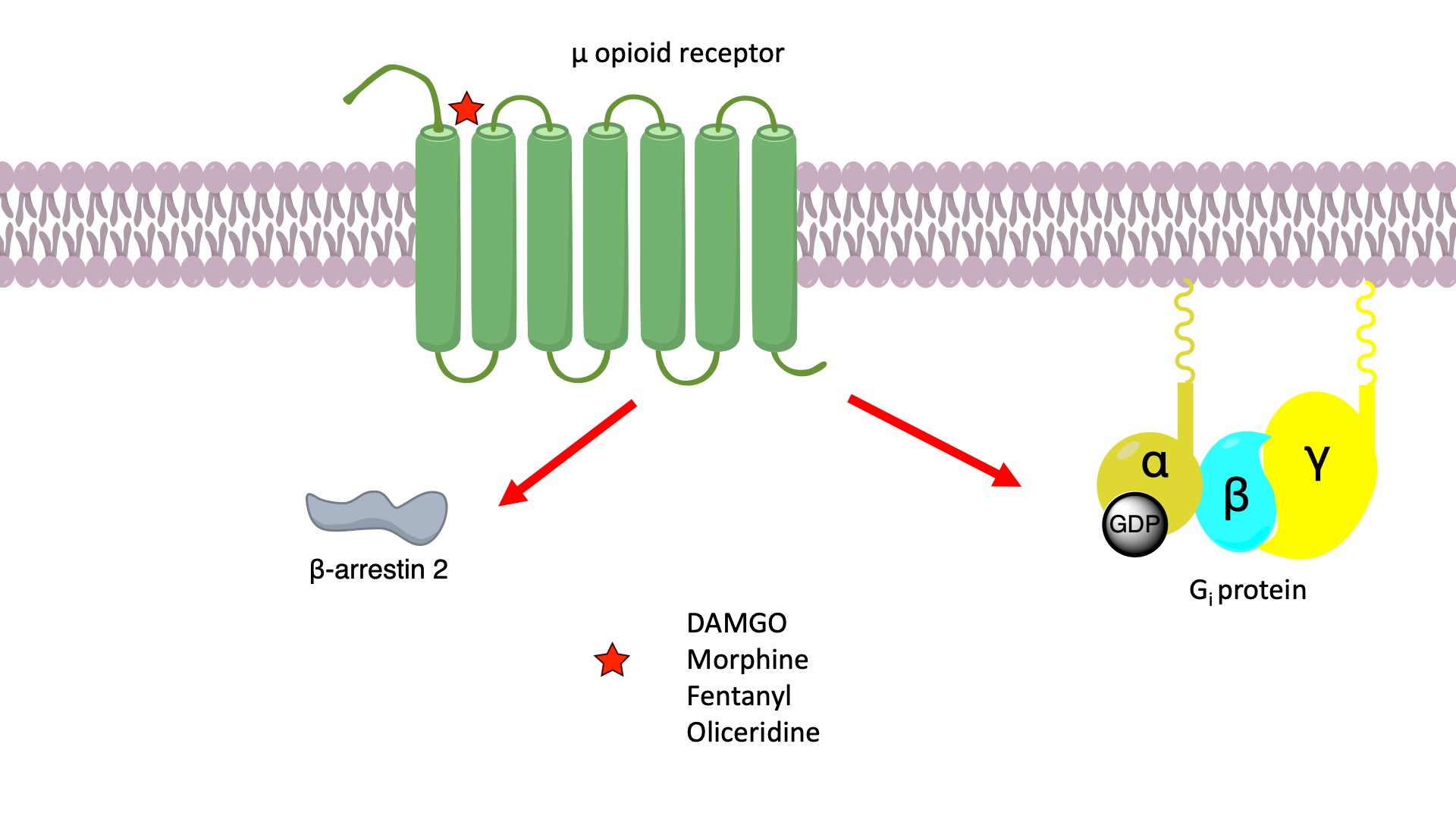
Signaling of the μ-opioid receptor is primarily transduced through Gi proteins to inhibit cAMP production. On the other hand, activation of the opioid receptor also leads to receptor phosphorylation by protein kinases such as GRK2. This triggers binding of beta-arrestin 2 to the receptor, which sterically inhibits further G protein activation and results in the activation of other downstream signaling pathways.
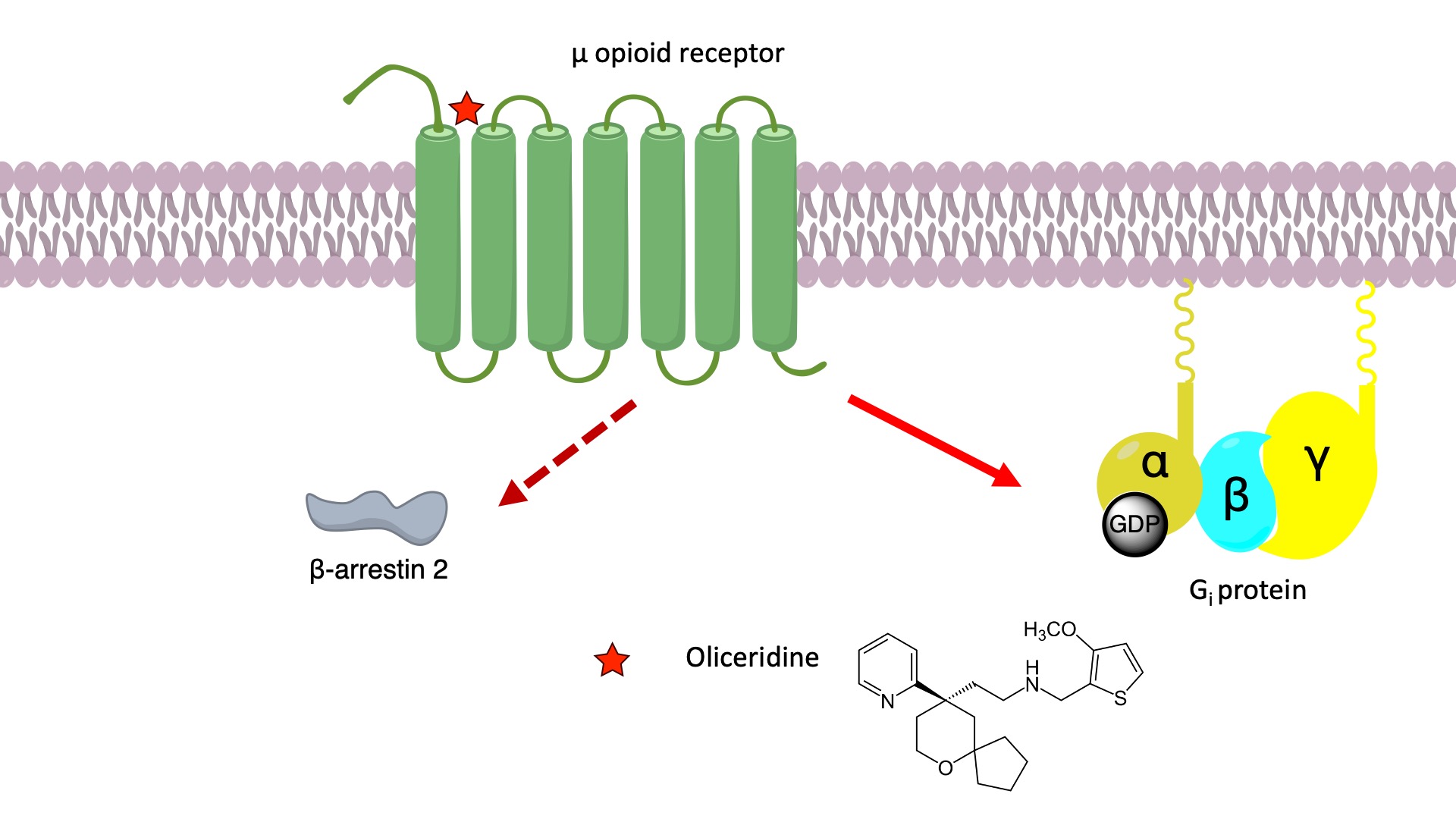
It has been proposed that the opioid-induced analgesia is attributed to Gi signaling, whereas the undesired side effects such as respiratory suppression and constipation might be caused by β-arrestin signaling. The proposed role of β-arrestins in the unwanted effects of opioid receptor agonists has led to the development of ligands that do not recruit β-arrestin 2 to the receptor, under the assumption that this would avoid on-target side effects mediated by the proposed β-arrestin–dependent mechanism. Oliceridine acts as a biased agonist at the μ-opioid receptor by preferentially activating the G-protein pathway with minimal receptor phosphorylation and recruitment of β-arrestin. [1]
It is proposed that oliceridine binding induces a different intracellular conformation of the μ-opioid receptor, due to a lack of coupling with transmembrane helix six and seven, which confers the specificity for G-protein over β-arrestin interaction. Therefore, ligands designed to reduce TM6/7 interactions display preferential G protein signaling. [2]
Some studies compared the efficacy of opioid agonists such as fentanyl, morphine, and oliceridine, using an analog of the endogenous opioid peptide, DAMGO, which was used as a reference, and arrived at the conclusion that oliceridine displayed lower intrinsic efficacy, acting as a partial agonist. No evidence of statistically significant bias was observed toward G protein signaling compared to β-arrestin 2 recruitment and concluded that low-efficacy agonists, such as oliceridine will produce strong responses in highly amplified G protein assays while having little activity in no-amplification, protein-protein interaction assays such as β-arrestin recruitment. [3]
Some recent studies also demonstrated that the μ-opioid agonists’ morphine and fentanyl induced respiratory depression and constipation in β-arrestin 2 knockout mice to the same extent as that observed in wild-type mice. These studies do not support the original suggestion that β-arrestin 2 signaling plays a key role in opioid-induced respiratory depression and call into question the concept of developing G protein-biased μ-opioid receptor agonists as a strategy for the development of safer opioid analgesic drugs. On the other hand, there are in vitro, in vivo, and clinical studies supporting the view that this biased agonism results in comparable analgesia compared with traditional opioids at a comparable or decreased risk of adverse effects. [4]
In any case, regardless of the mechanism of action, oliceridine is a potent compound that does exhibit to a lesser extent undesired side effects. Therefore, there is still room for improvement. And next-generation opioid drugs devoid of these undesired effects remain to be seen. [5]
Synthesis of Oliceridine
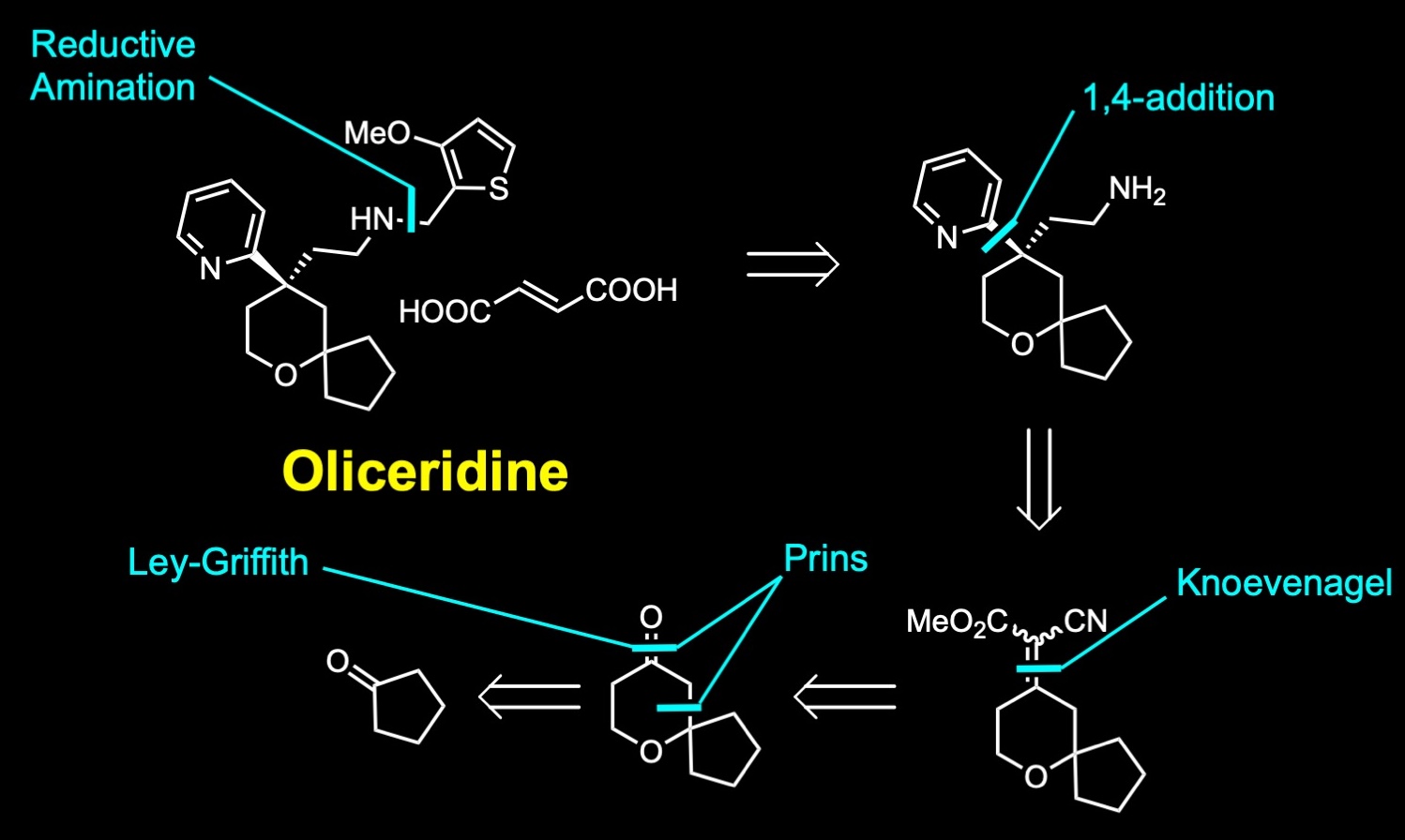
Oliceridine is a T-shaped chiral compound that presents a pyridine and a thiophene ring and a spirocycle. The synthesis of the molecule was first described by Trevena, and it involves a reductive amination. A 1,4-addition of the pyridyl ring to this intermediate followed by decarboxylation and reduction of the nitrile to the primary amine. A Knoevenagel condensation reaction. And a Ley-Griffith oxidation reaction to the alcohol obtained from a Prins cyclization.
Forward Synthesis of Oliceridine
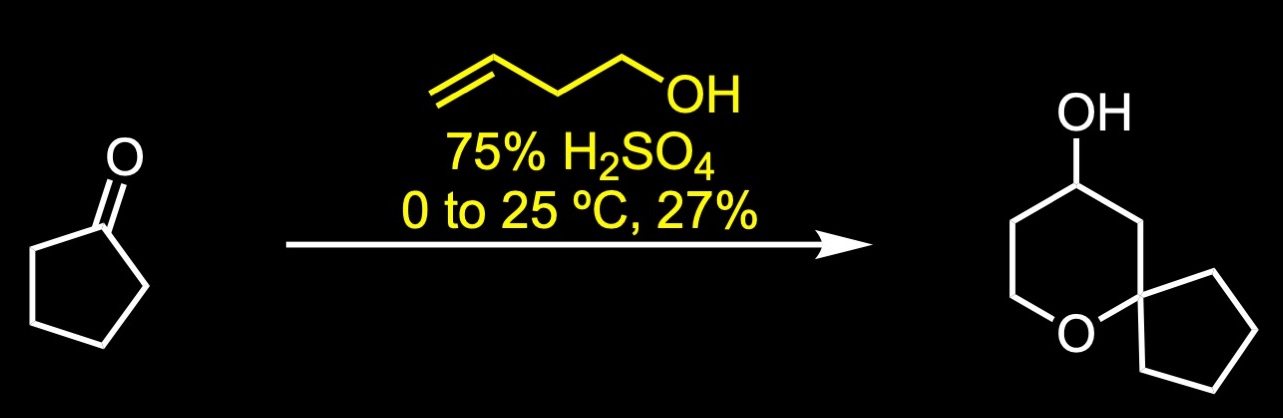
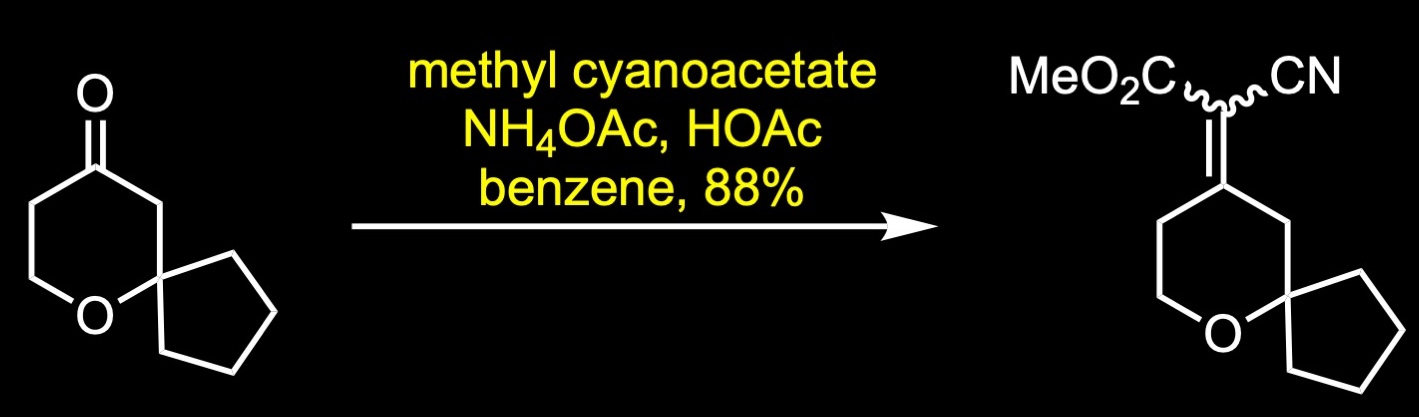
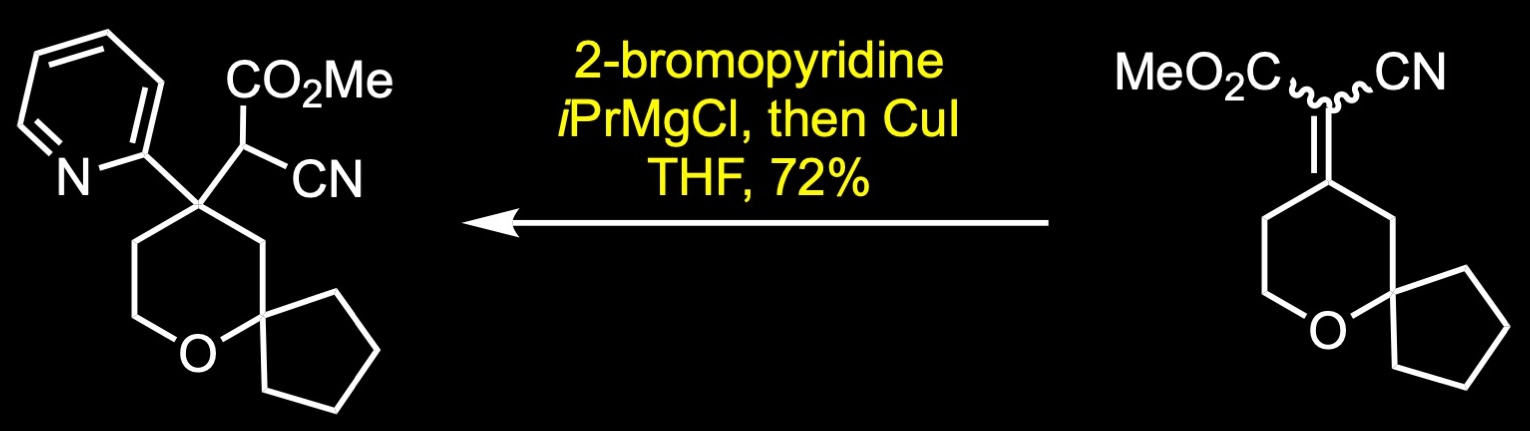
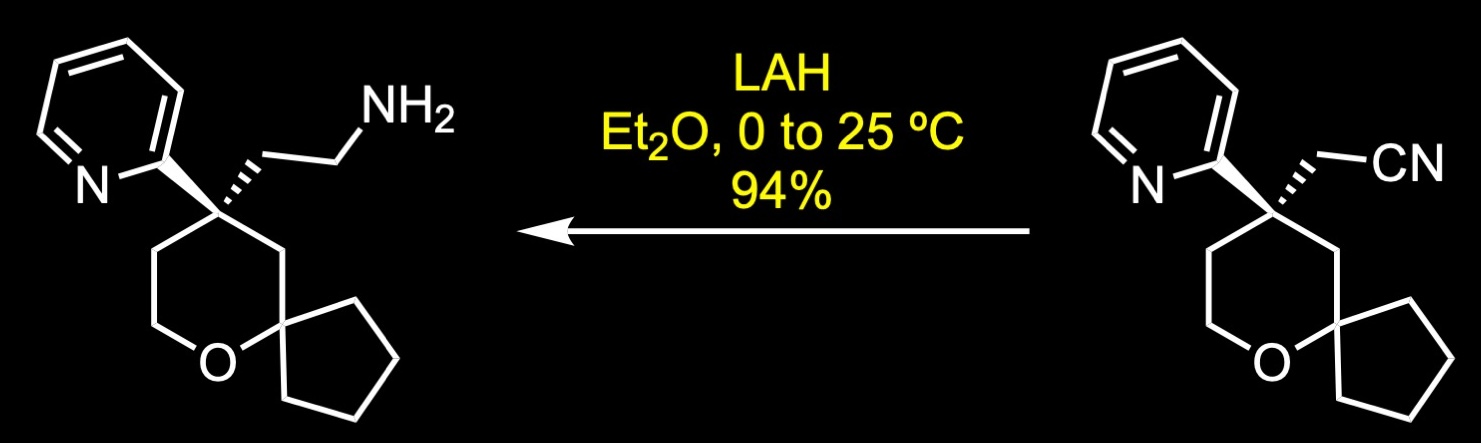
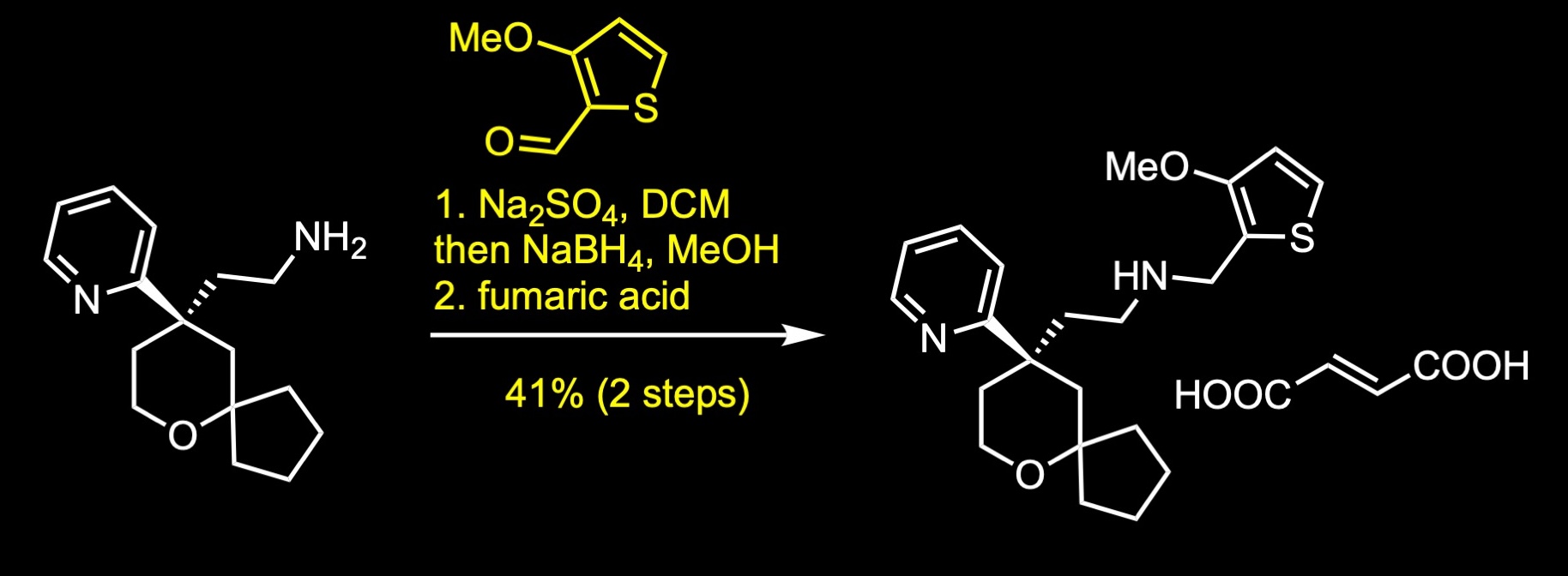
The forward synthesis begins with a Prins reaction between pentanone and butenol in 75% sulfuric acid. The Prins reaction is the acid-catalyzed condensation of alkenes with aldehydes or ketones. The reaction involves the electrophilic addition of a ketone to an alkene followed by the capture of water. The resulting alcohol was further oxidized to the corresponding ketone via Ley-Griffith oxidation. The Ley-Griffith reaction is the oxidation of primary or secondary alcohols to aldehydes or ketones. The central catalyst is tetra propylammonium perruthenate which is used in combination with the co-oxidant N-methylmorpholine N-oxide. Knoevenagel condensation between the cyclic ketone and methyl cyanoacetate provided a mixture of regioisomers, which were not separated. The aryl Grignard reagent was then added to the unsaturated cyanopropenoate in the presence of catalytic amount of copper iodide to give the conjugate addition product. Decarboxylation was achieved under strong basic conditions with heating to afford the nitrile, which was subsequently separated by SFC chiral separation. Reduction of the nitrile to its primary amine was accomplished with lithium aluminum hydride in ether. Reductive amination with the commercial aldehyde and fumarate salt formation provided oliceridine fumarate in 41% yield over two steps.
References
1. J. Med. Chem. 2013, 56, 8019.
2. Cell. 2022, 185, 4361. Open access.
3. Sci. Signal. 13, eaaz3140 (2020)
4. Br J Pharmacol. 2020, 177, 2923. Open access.
5. Mol Pharmacol. 2019, 96, 542.
6. J. Med. Chem. 2022, 65, 9607.
Oliceridine-bound mu-opioid receptor-Gi complex: PDB ID: 8EFB.