Negishi Coupling
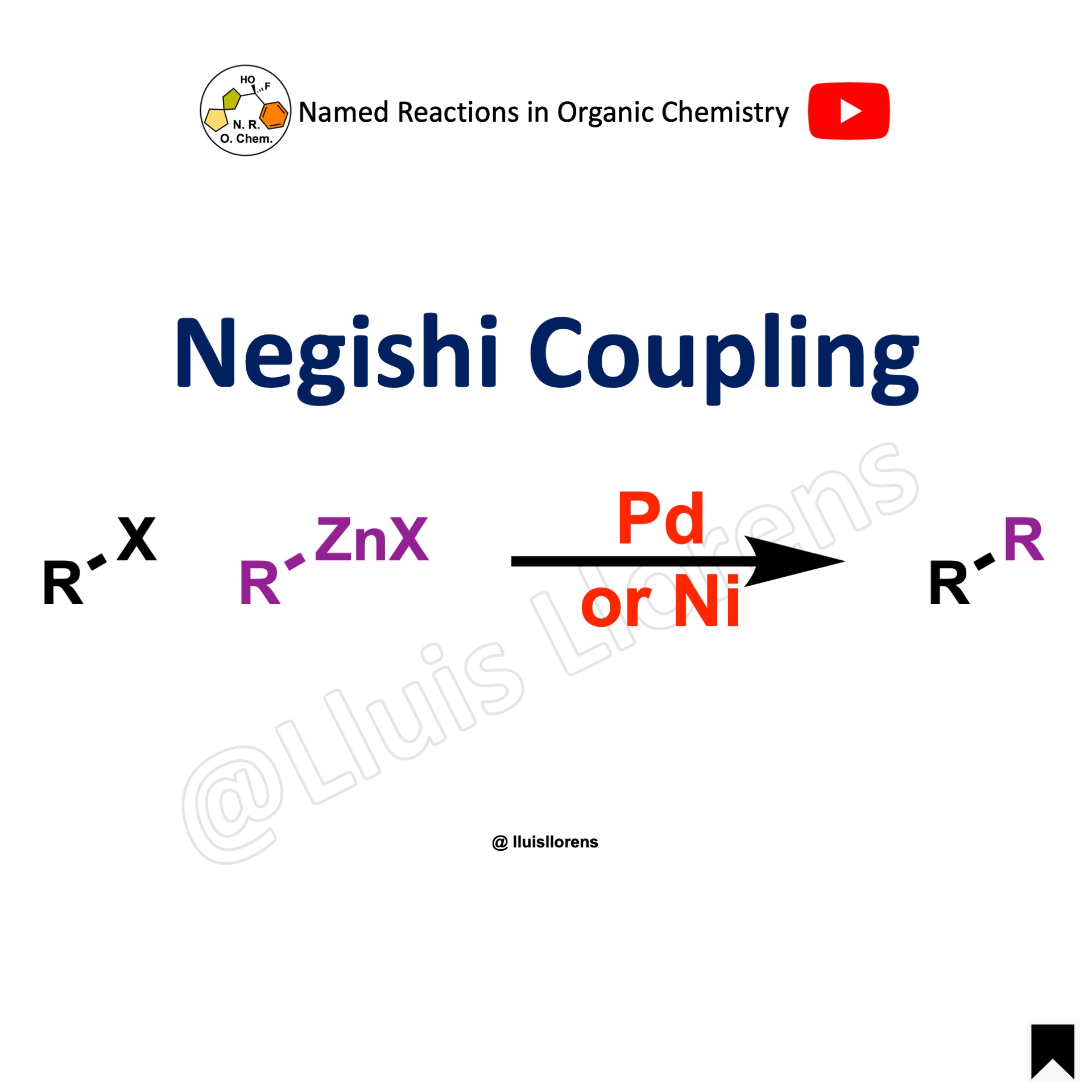
The Negishi coupling is the nickel or palladium-catalyzed stereoselective reaction of organozincs and organic halides for the formation of carbon-carbon bonds. Even though, organozincs are more reactive than organostannanes (for the Stille coupling) and organoboranes (for the Suzuki coupling), they are moisture and air sensitive, so the Negishi coupling must be performed in an oxygen and water free environment.
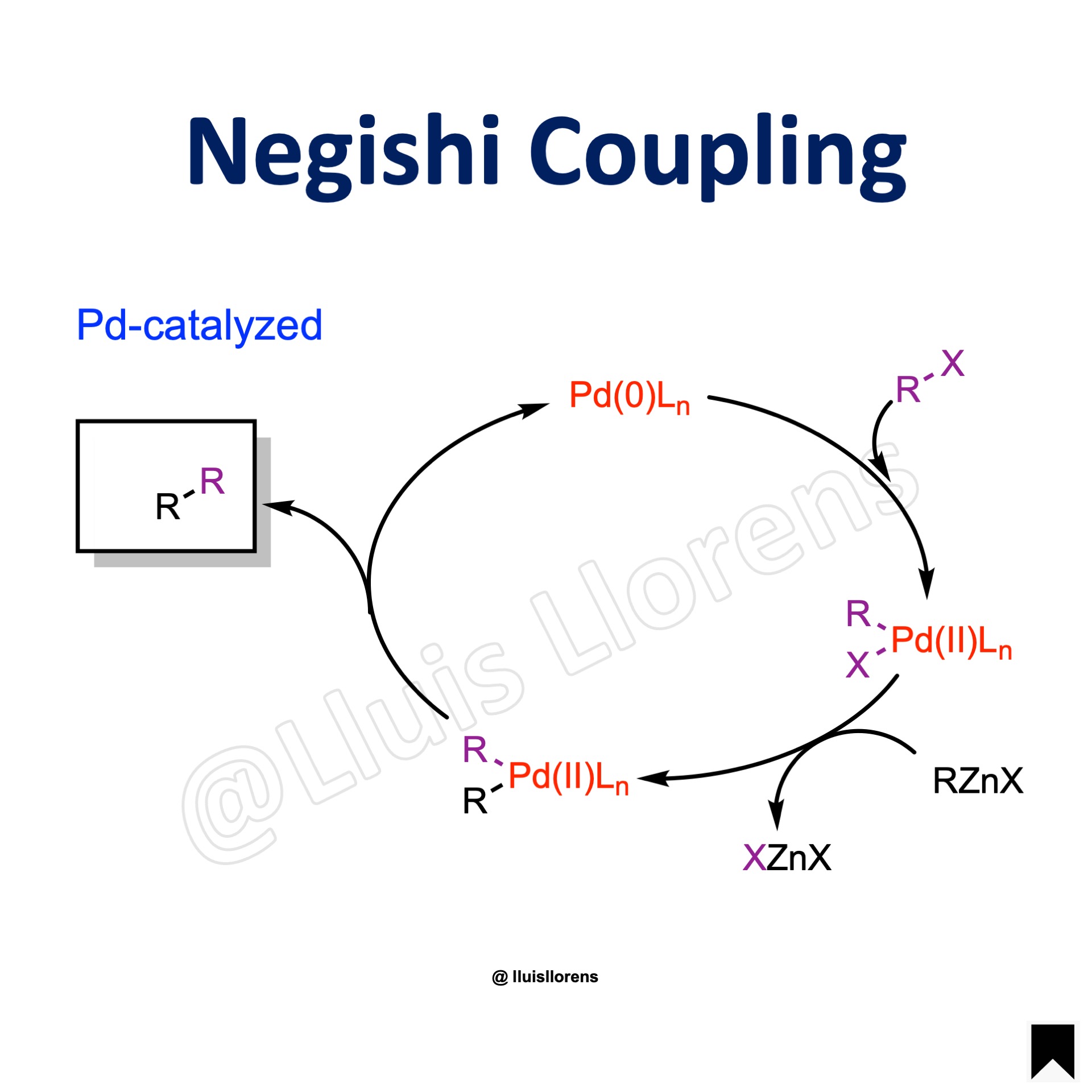
Reaction Mechanism
1. Initially, the electron-rich Pd(0) catalyst inserts into the R–X bond of the organic halide. This oxidative addition forms an organo-Pd(II)-complex. 2. Subsequent transmetalation with the organozinc reagent forms a hetero organometallic complex. 3. Before the next step, isomerization is necessary to bring the organic ligands next to each other into cis positions. (Not shown.) 4. Finally, reductive elimination leads to the formation of a C–C bond and releases the cross coupled product while regenerating the Pd(0) catalyst.
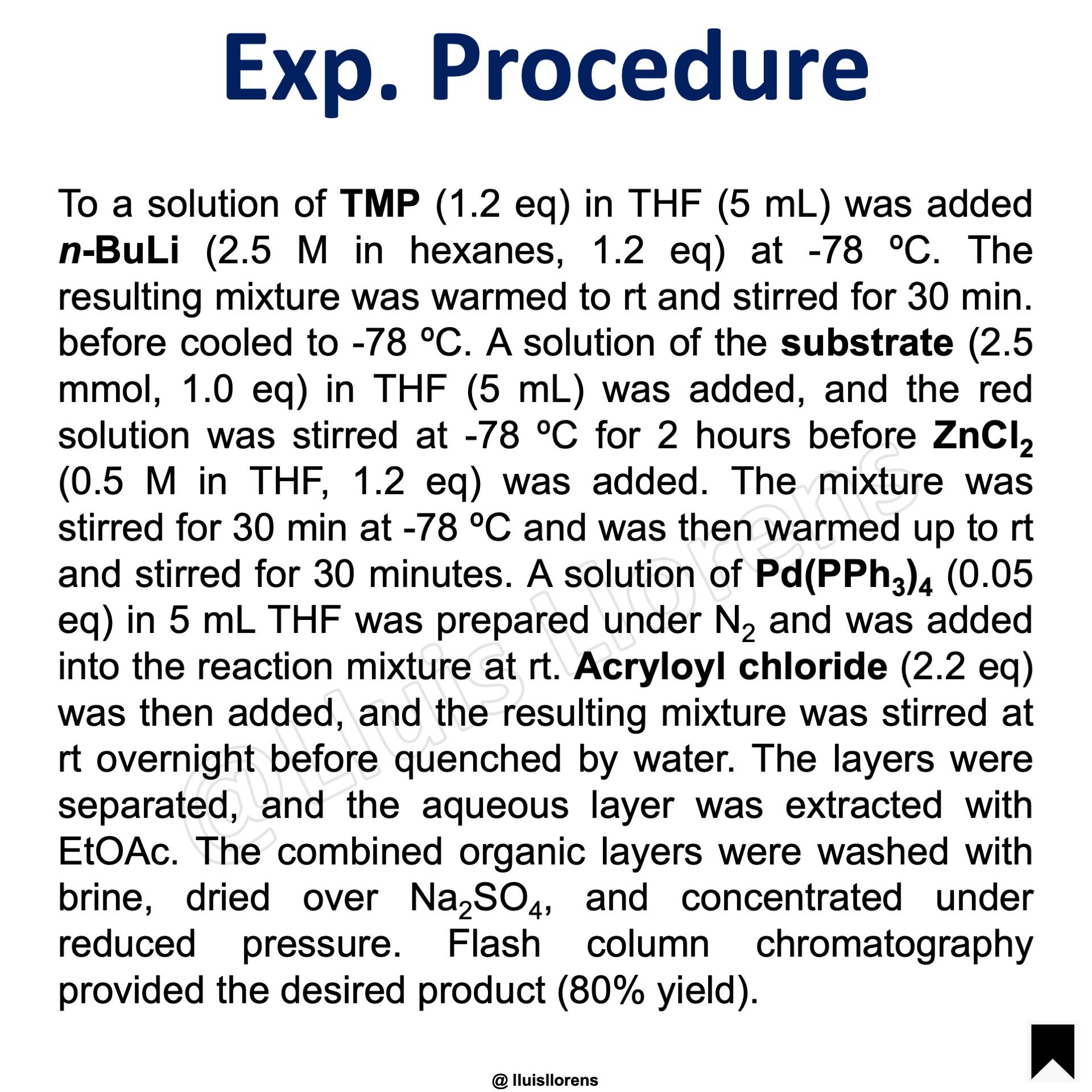
Experimental Procedure
To a solution of TMP (1.2 eq) in THF (5 mL) was added n–BuLi (2.5 M in hexanes, 1.2 eq) at -78 ºC. The resulting mixture was warmed to rt and stirred for 30 min. before cooled to -78 ºC. A solution of the substrate (2.5 mmol, 1.0 eq) in THF (5 mL) was added, and the red solution was stirred at -78 ºC for 2 hours before ZnCl2 (0.5 M in THF, 1.2 eq) was added. The mixture was stirred for 30 min at -78 ºC and was then warmed up to rt and stirred for 30 minutes. A solution of Pd(PPh3)4 (0.05 eq) in 5 mL THF was prepared under N2 and was added into the reaction mixture at rt. Acryloyl chloride (2.2 eq) was then added, and the resulting mixture was stirred at rt overnight before quenched by water. The layers were separated, and the aqueous layer was extracted with EtOAc. The combined organic layers were washed with brine, dried over Na2SO4, and concentrated under reduced pressure. Flash column chromatography provided the desired product (80% yield).
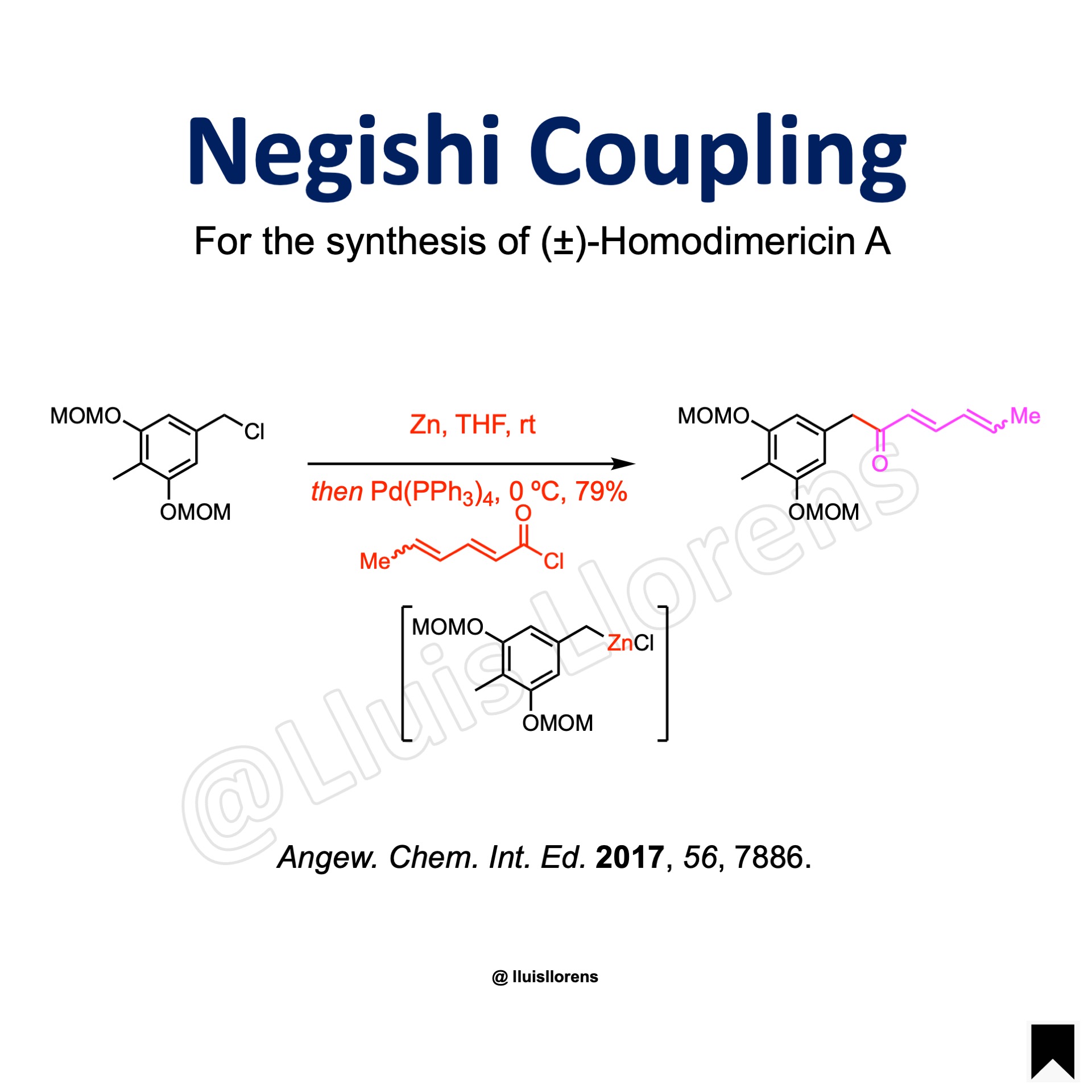
More Examples
Learn More Named Reactions
[instagram-feed feed=2]