Nazarov Cyclization
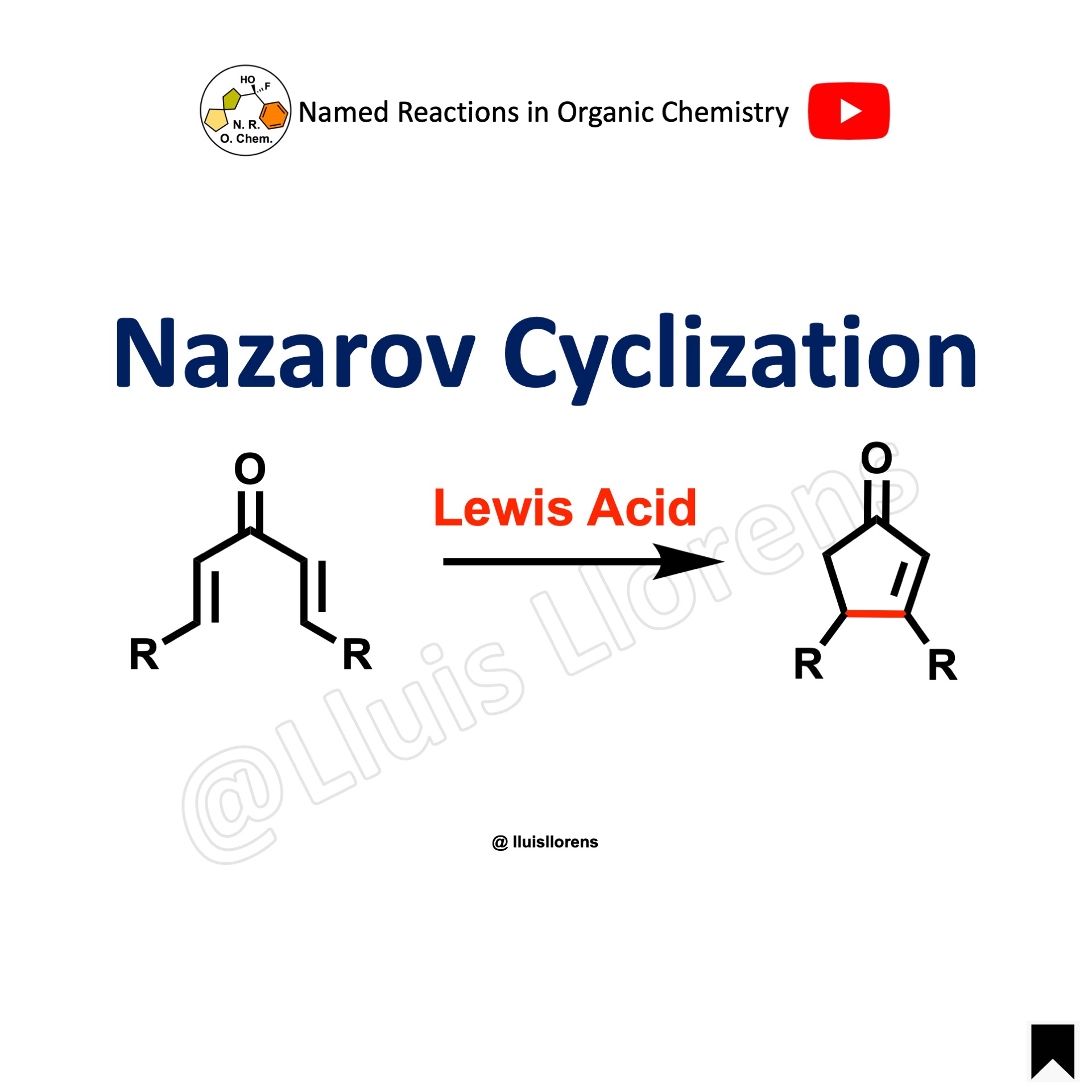
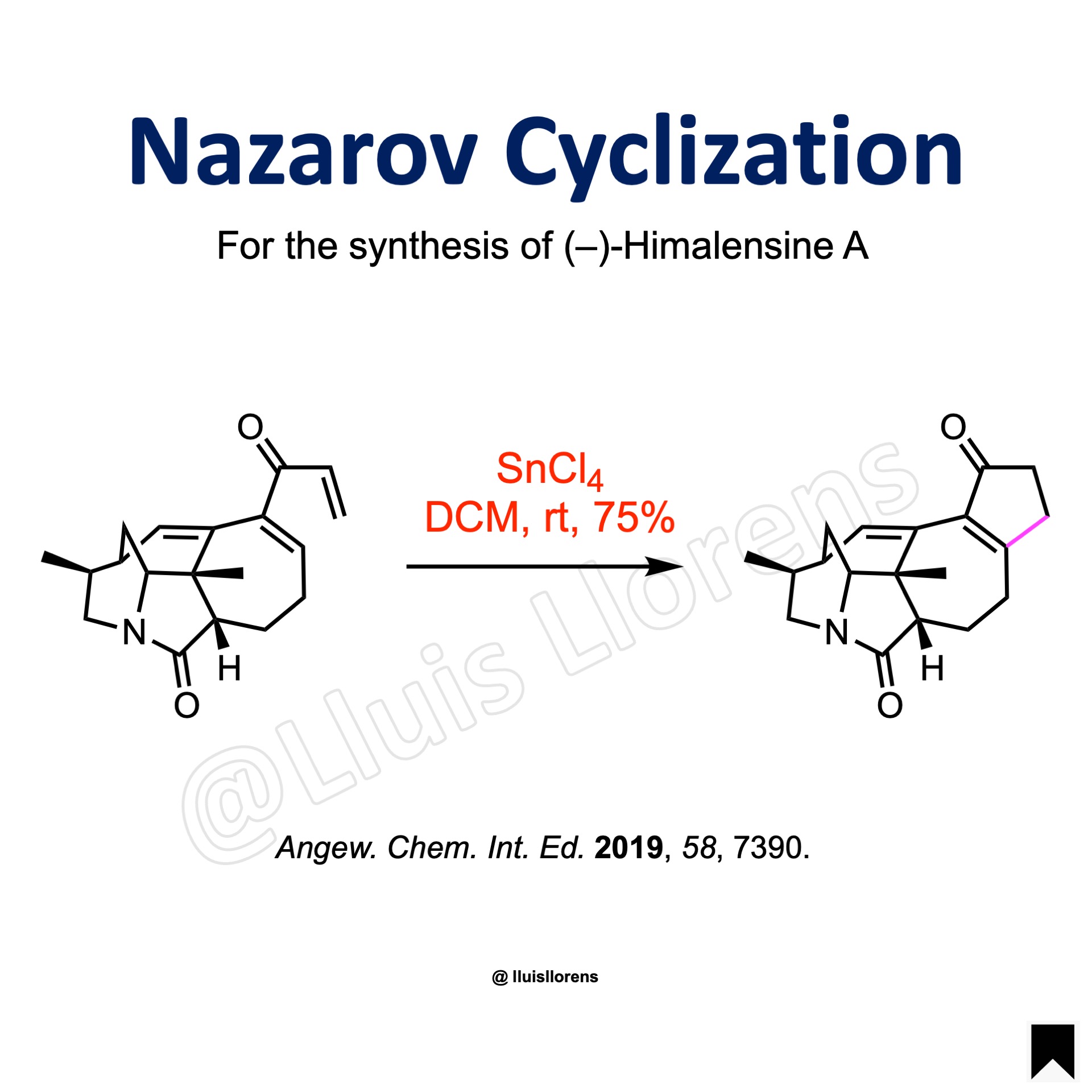
The Nazarov cyclization is the acid catalyzed ring-closure of divinyl ketones for the synthesis of cyclopentenones.
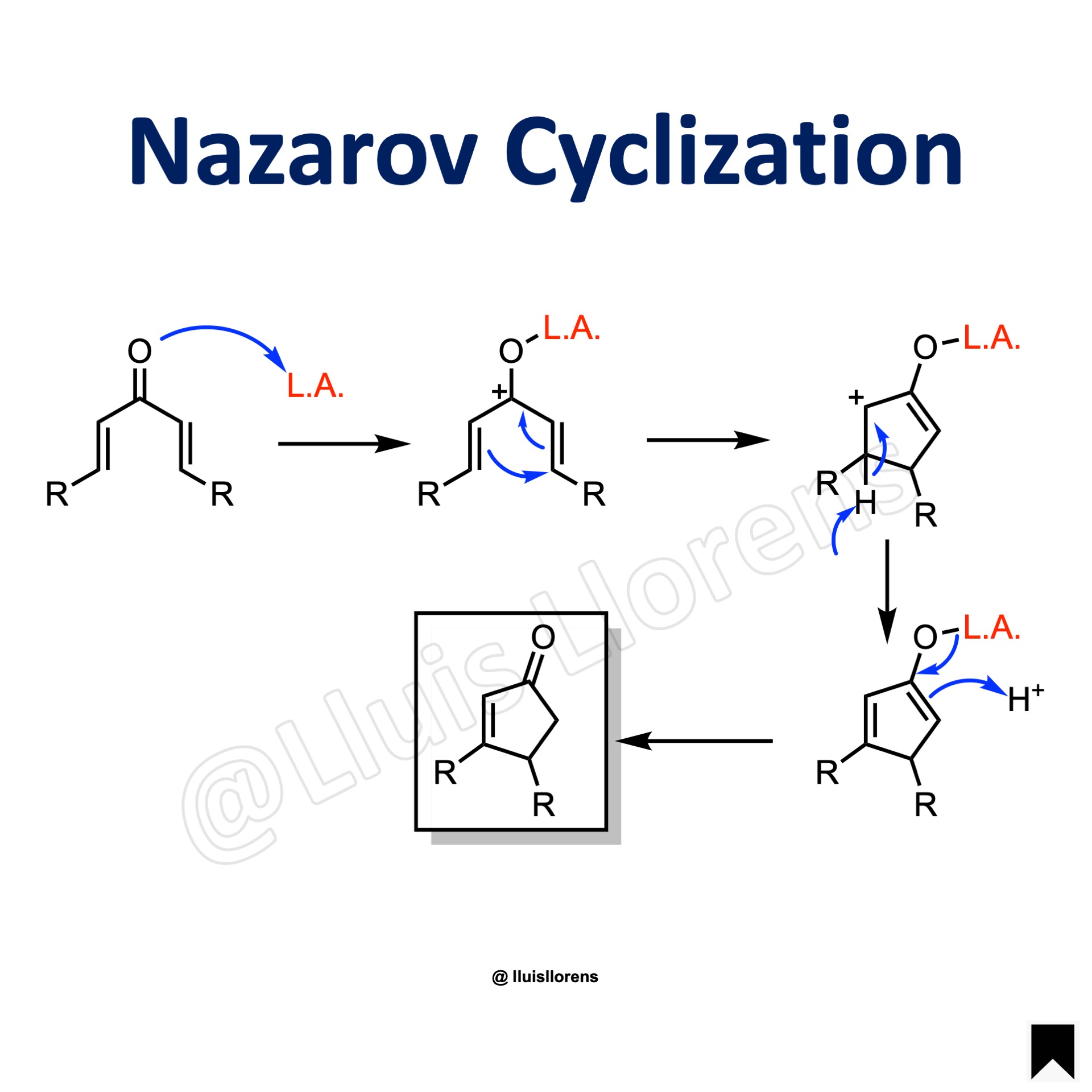
Reaction Mechanism
1. Coordination of the Lewis acid to the carbonyl group and formation of a pentadienyl cation. 2. Conrotatory ring closure delivers a cyclic carbocation. 3. Elimination of the β-hydrogen. 4. Tautomerization of the enolate delivers the product.
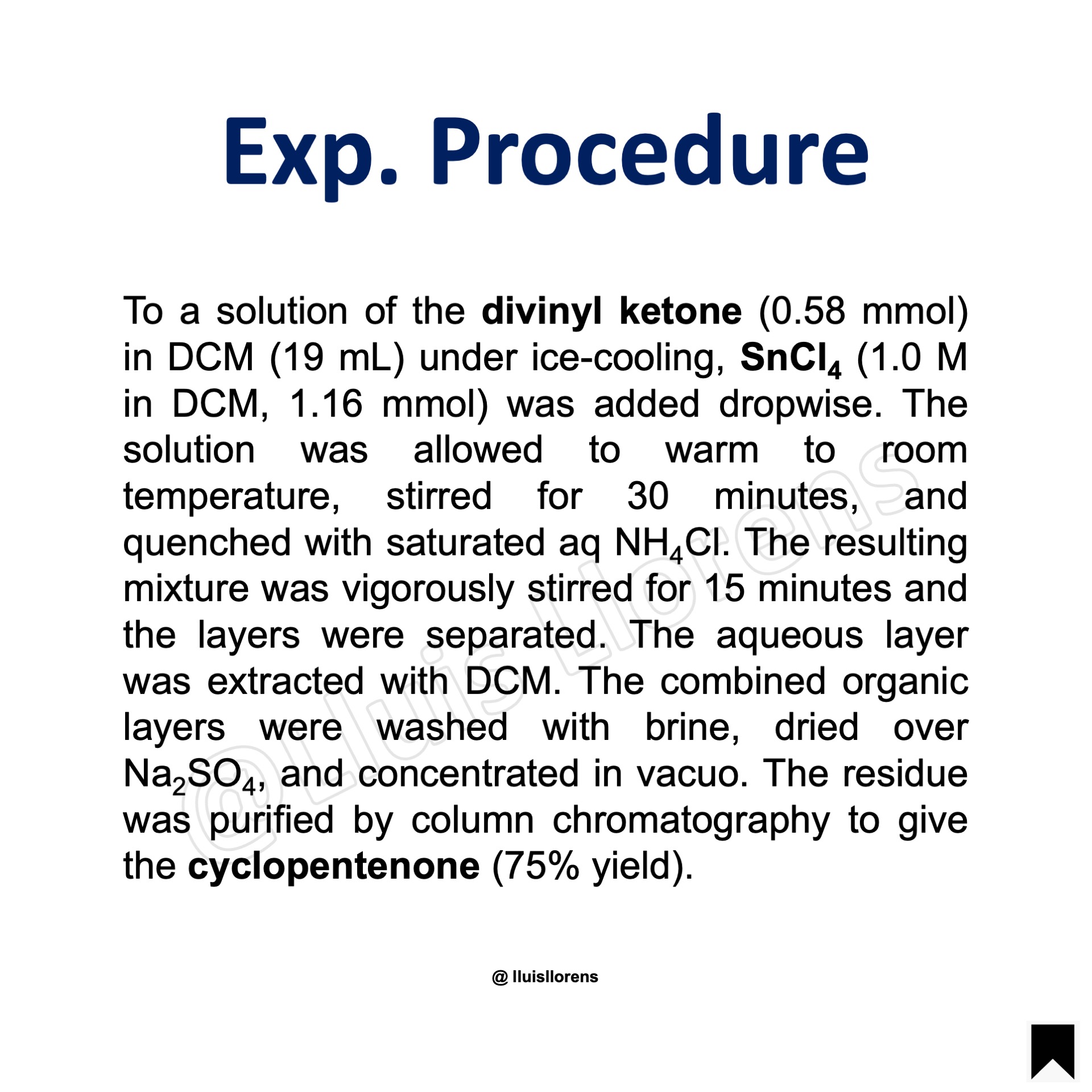
Experimental Procedure
To a solution of the divinyl ketone (0.58 mmol) in DCM (19 mL) under ice-cooling, SnCl4 (1.0 M in DCM, 1.16 mmol) was added dropwise. The solution was allowed to warm to room temperature, stirred for 30 minutes, and quenched with saturated aq NH4Cl. The resulting mixture was vigorously stirred for 15 minutes and the layers were separated. The aqueous layer was extracted with DCM. The combined organic layers were washed with brine, dried over Na2SO4, and concentrated in vacuo. The residue was purified by column chromatography to give the cyclopentenone (75% yield).
Learn More Named Reactions
[instagram-feed feed=2]