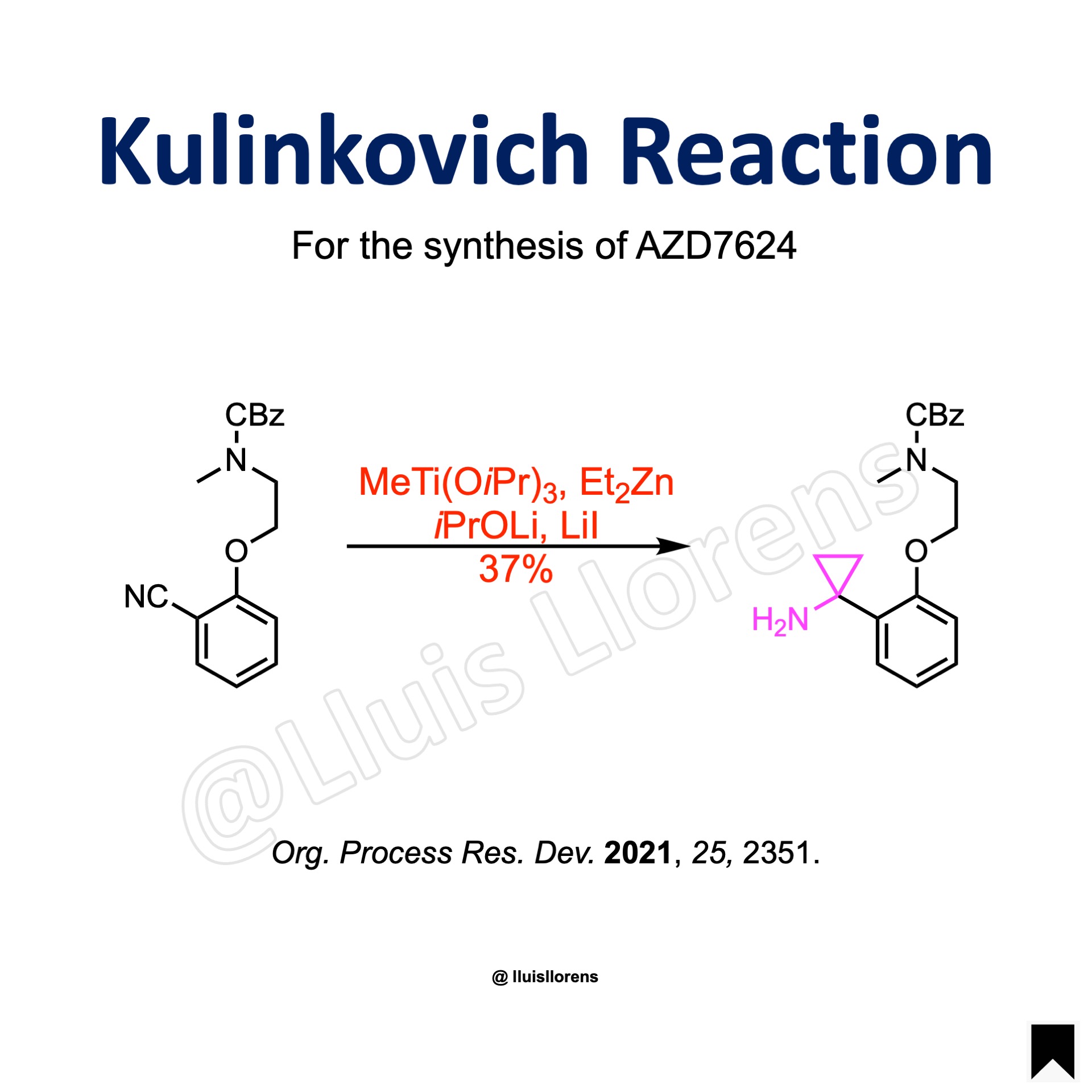
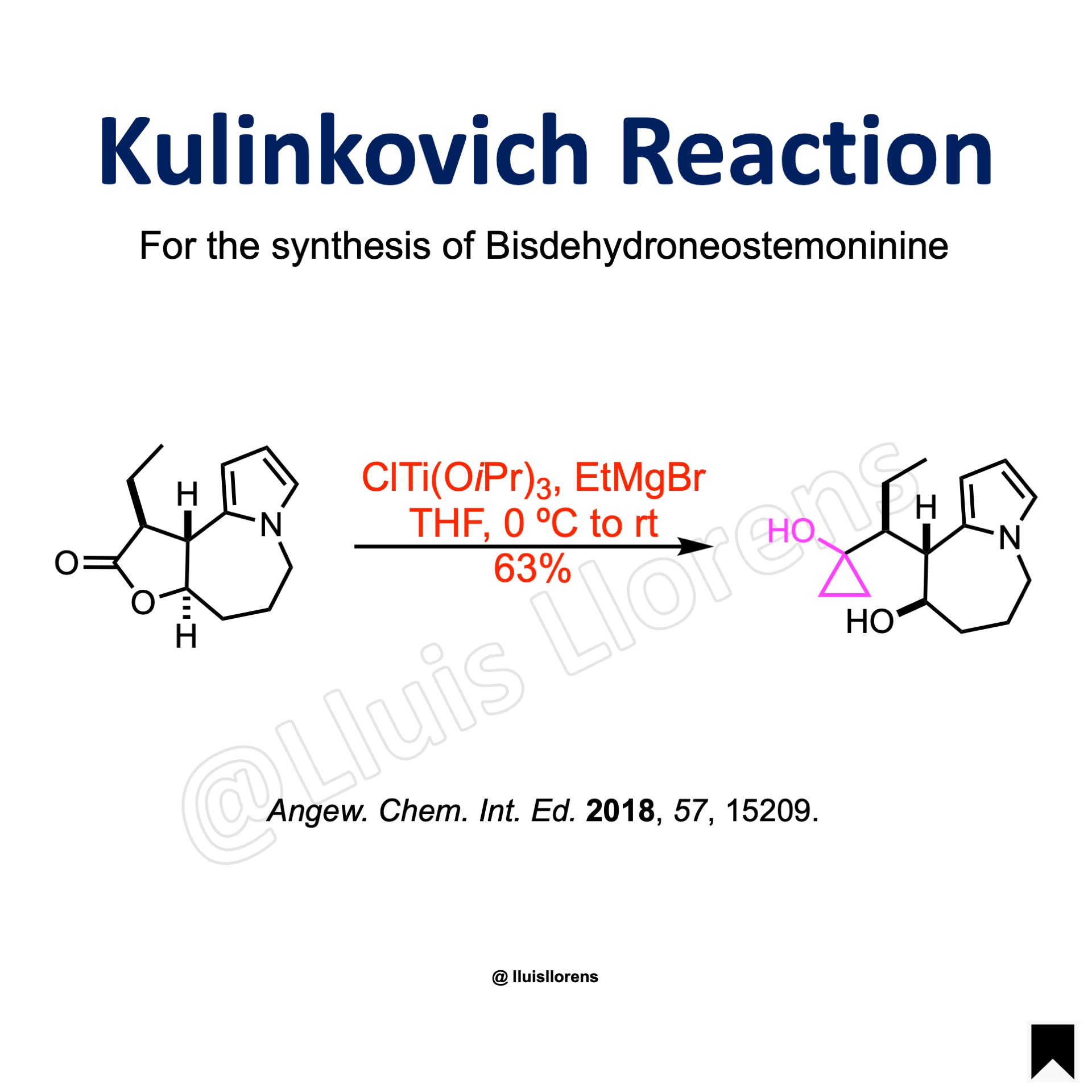
Kulinkovich Reaction
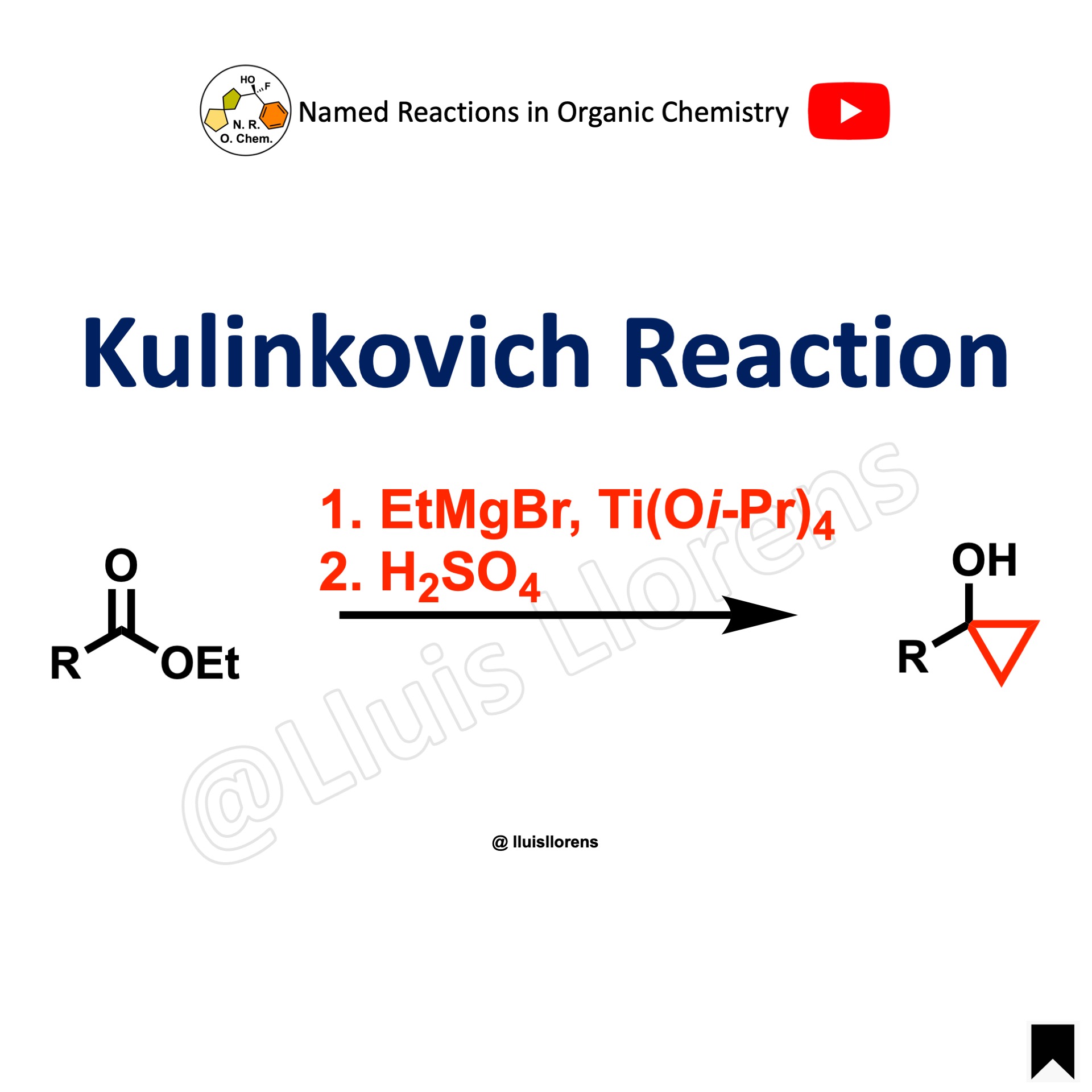
The Kulinkovich reaction allows the preparation of cyclopropanol derivatives by the reaction of Grignard reagents with carboxylic esters in the presence of titanium(IV) isopropoxide.
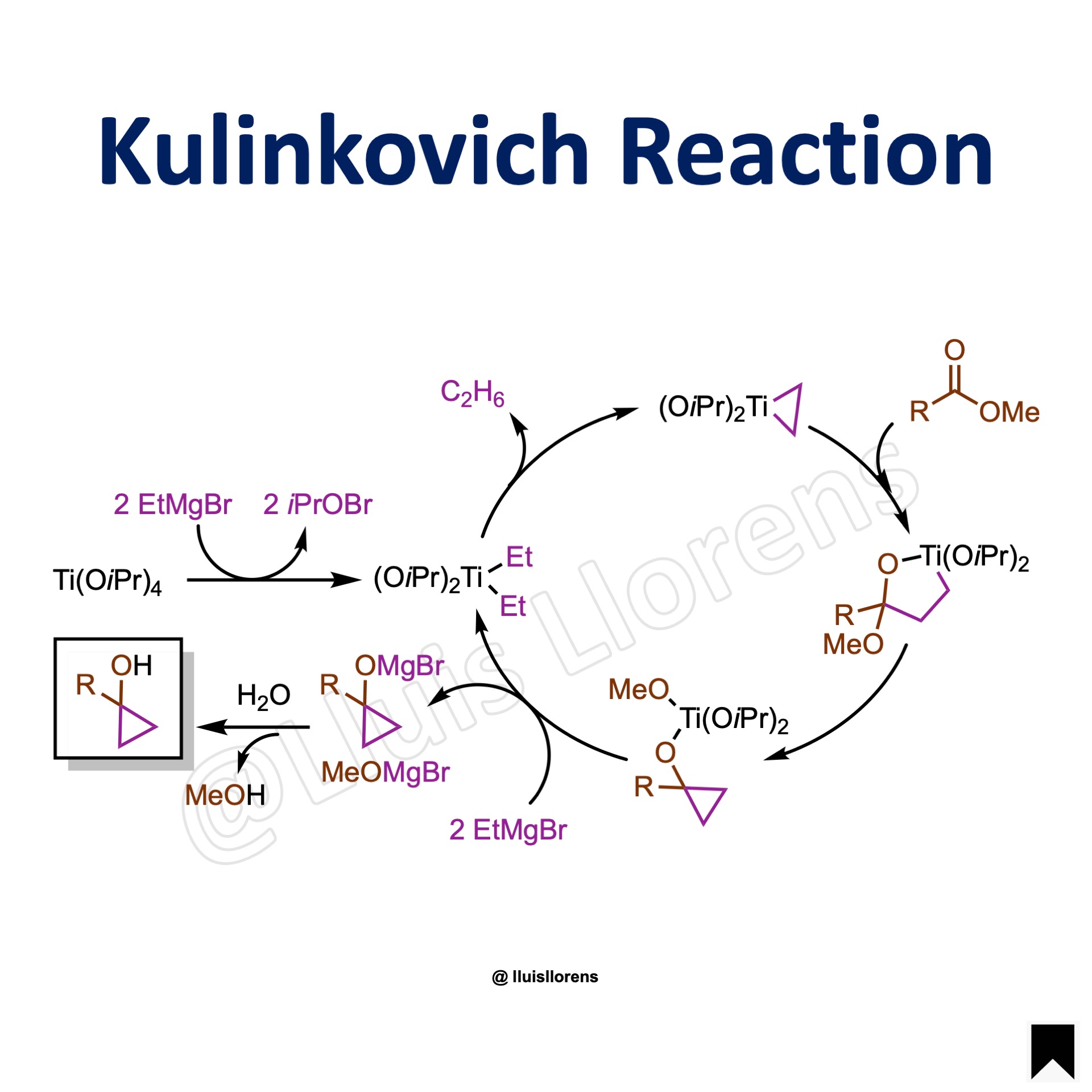
Reaction Mechanism
1. Dialkylation of the titanium catalyst with two equivalents of ethylmagnesium bromide. 2. β-hydride elimination gives ethane and titanacyclopropane. 3. This titanacyclopropane reacts with the carboxylic ester to produce a cyclopropanol derivative. 4. Addition of ethylmagnesium bromide to the titanium complex delivers the magnesium cyclopropoxide product while regenerating the diethyltitanium intermediate. 5. Aqueous/acidic workup yields the 1-cyclopropanol product.
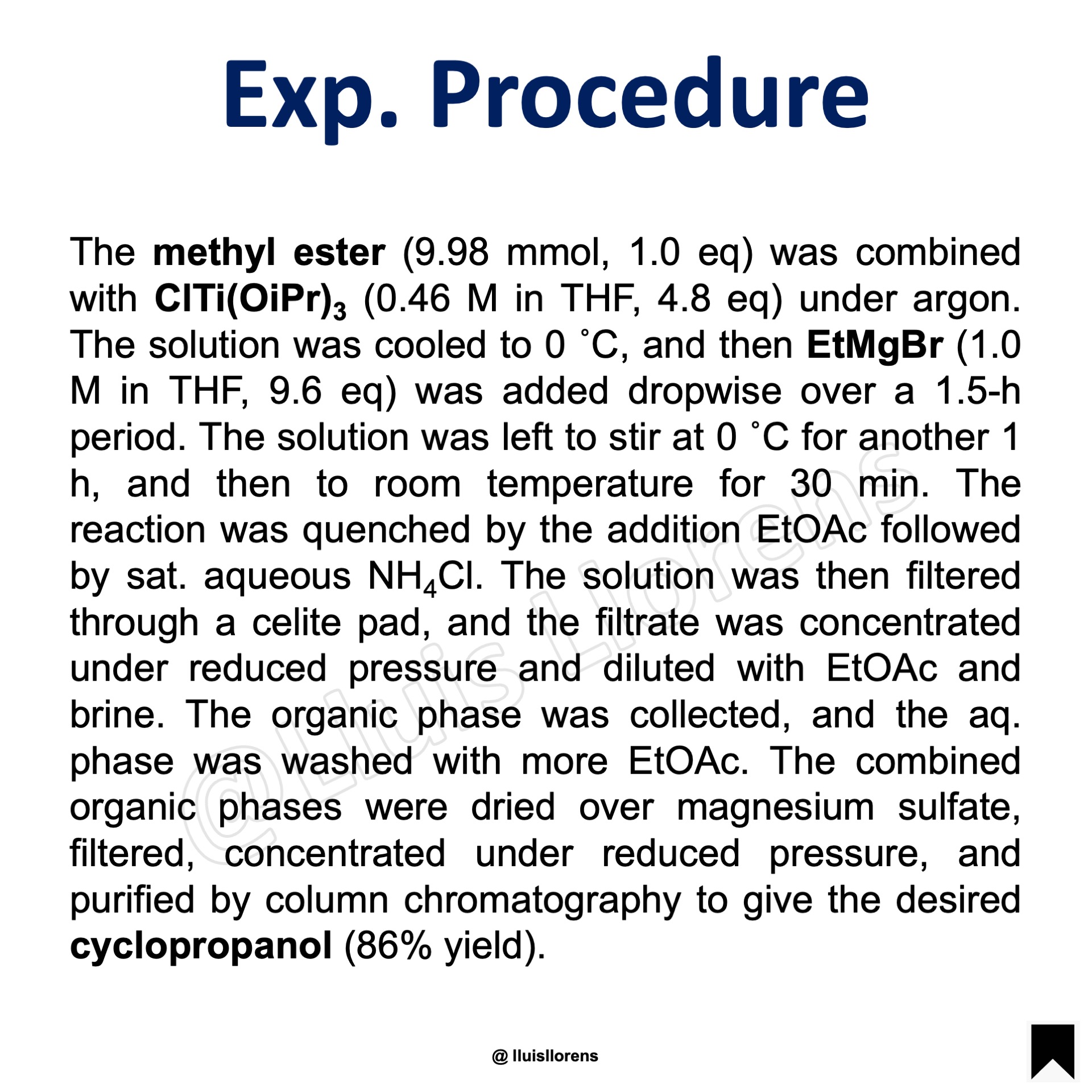
Experimental Procedure
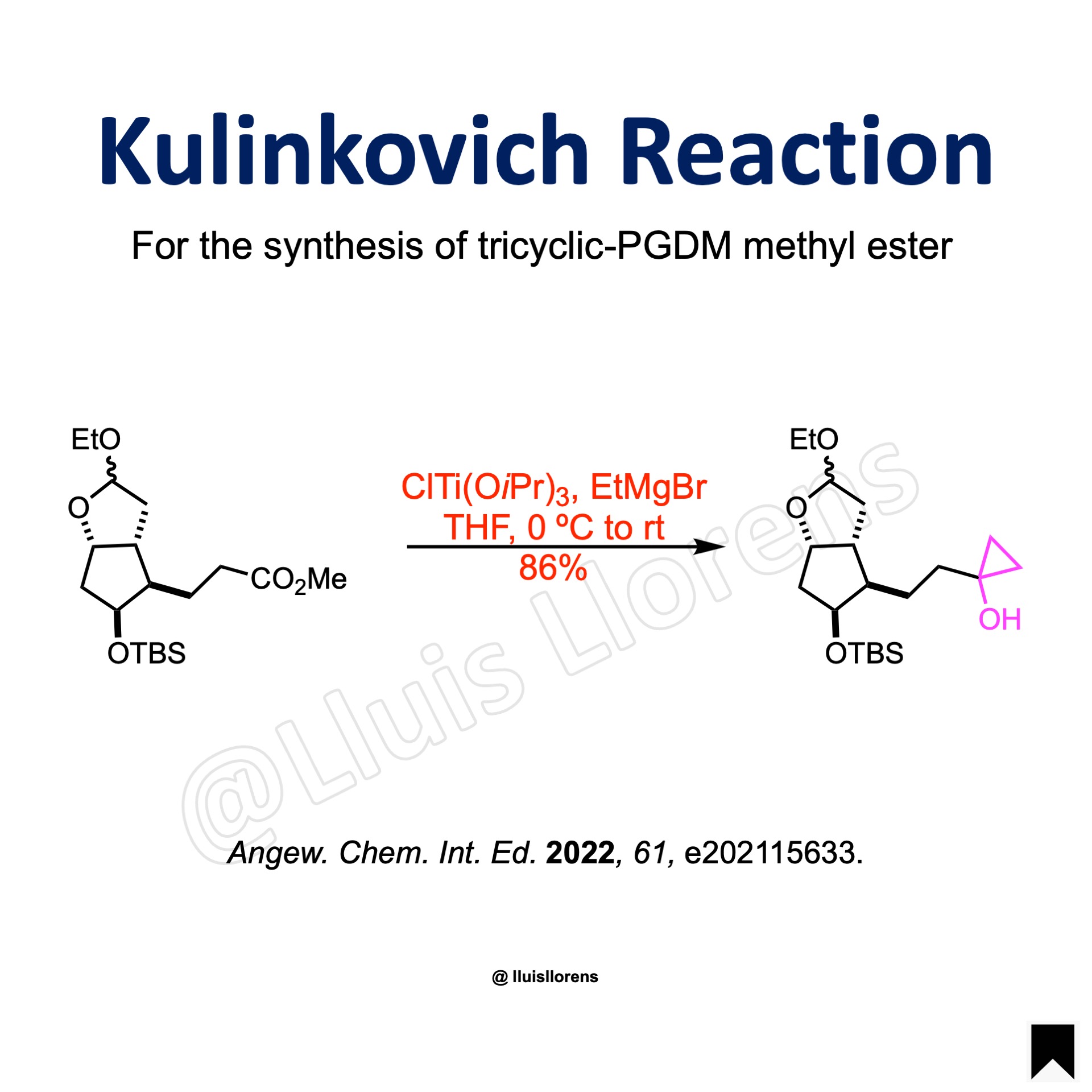
The methyl ester (9.98 mmol, 1.0 eq) was combined with ClTi(OiPr)3 (0.46 M in THF, 4.8 eq) under argon. The solution was cooled to 0 ˚C, and then EtMgBr (1.0 M in THF, 9.6 eq) was added dropwise over a 1.5-h period. The solution was left to stir at 0 ˚C for another 1 h, and then to room temperature for 30 min. The reaction was quenched by the addition EtOAc followed by sat. aqueous NH4Cl. The solution was then filtered through a celite pad, and the filtrate was concentrated under reduced pressure and diluted with EtOAc and brine. The organic phase was collected, and the aq. phase was washed with more EtOAc. The combined organic phases were dried over magnesium sulfate, filtered, concentrated under reduced pressure, and purified by column chromatography to give the desired cyclopropanol (86% yield).
Learn More Named Reactions
[instagram-feed feed=2]