Ireland-Claisen Rearrangement
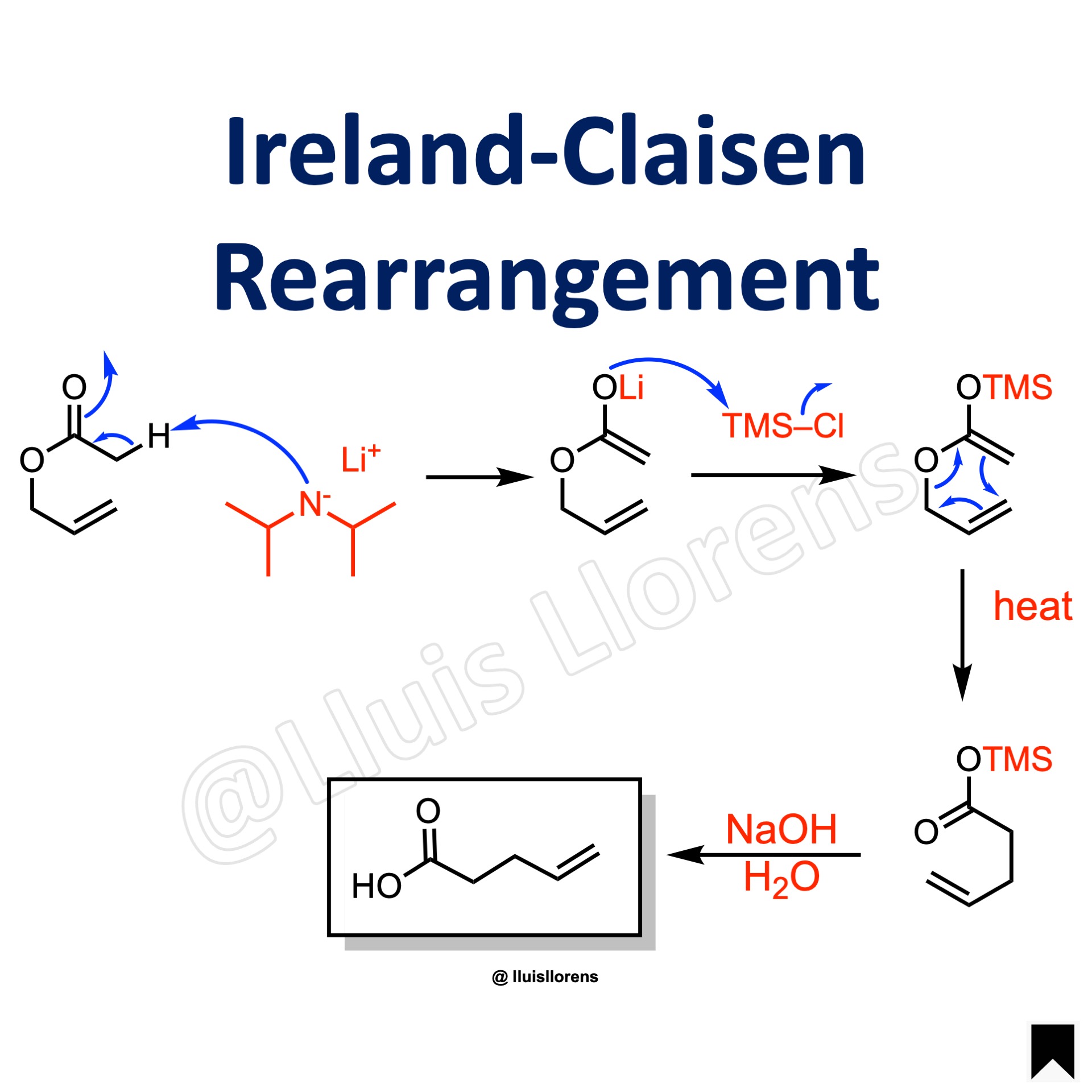
The Ireland-Claisen rearrangement is the [3,3]-sigmatropic rearrangement of a silyl-stabilized enolate (silyl ketene acetal) derivatized from an allylic ester with a strong base to deliver a gamma,delta-unsaturated carboxylic acid.
General features:
1. The reaction allows the conversion of a carbon-oxygen bond into a carbon-carbon bond. 2. The rearrangement takes place under much milder conditions than the regular Claisen rearrangement. 3. Deprotonation with LDA/THF leads to the kinetically favored (Z)-ester enolates. In the presence of THF/HMPA, the (E)-ester enolates are formed instead.
Reaction Mechanism
1. Formation of the corresponding enolate upon deprotonation with LDA. 2. The enolate is trapped with a trialkylsilyl halide. 3. [3,3]-sigmatropic rearrangement. 4. Hydrolysis.
Experimental Procedure
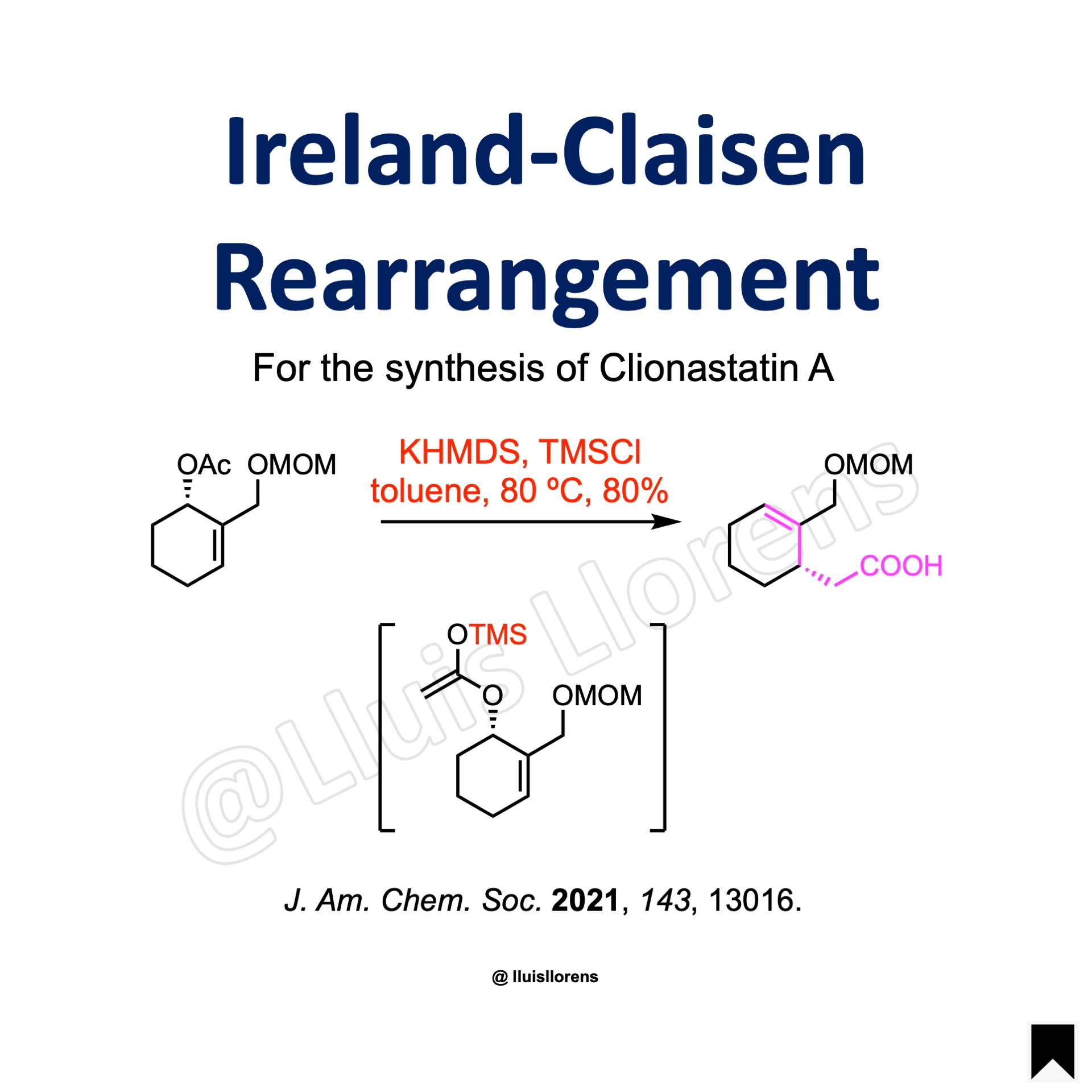
To a solution of the allylic ester (46.7 mmol, 1.0 eq) in 650 mL toluene was added KHMDS (1.0 M in THF, 2.0 eq) slowly at -78 ℃. After stirring at this temperature for 30 min, TMSCl (2.5 eq) was added. After warmed to room temperature and stirred for another 80 min, the reaction mixture was heated at 80 ℃ for 4 h before it was quenched with 0.5 N aq. HCl. The resultant mixture was extracted with EtOAc, and the combined organic layers were washed with brine, dried over anhydrous Na2SO4 and filtered. The volatiles were removed under vacuum, and the residue was purified by flash column chromatography to give the γ,δ–unsaturated carboxylic acid (80% yield).
Learn More Named Reactions
[instagram-feed feed=2]