Hell-Volhard-Zelinsky Reaction
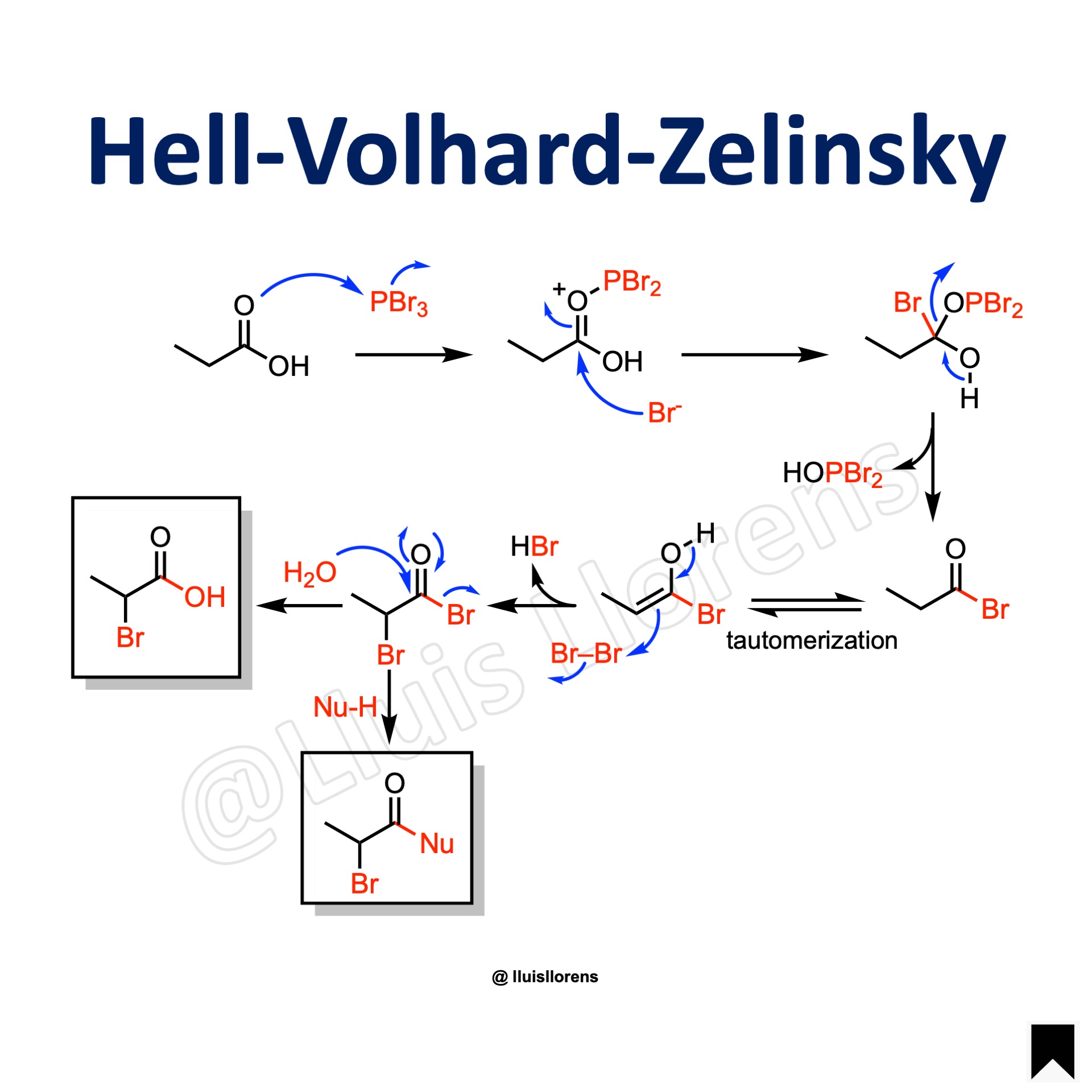
The Hell-Volhard-Zelinsky reaction allows the preparation of alpha-halo carboxylic acids by treating the corresponding acid with an halogen and phosphorous trihalide at high temperatures.
General features: 1. The initial product of the HVZ reaction is an α-halo acyl halide, which usually is hydrolyzed to the corresponding α-halo acid during the aqueous work-up. However, in the presence of nucleophiles such as alcohols, thiols, and amines, the corresponding α-halo esters, thioesters, and amides are formed instead. 2. Harsh reaction conditions: high temperatures and extended reaction times. 3. In addition to bromide, chloride also undergoes alpha-chlorination. The fluorination or iodination of carboxylic acids under HVZ conditions is not feasible.
Reaction Mechanism
1. The carboxylic acid reacts with phosphorous tribromide. 2. Formation of an acyl bromide. 3. Tautomerization to the enol form and reaction with bromine. 4. Hydrolysis yields the alpha-bromo carboxylic acid. 4.1. By quenching the reaction with an alcohol, instead of water, the α-bromo ester can be obtained.
Experimental Procedure
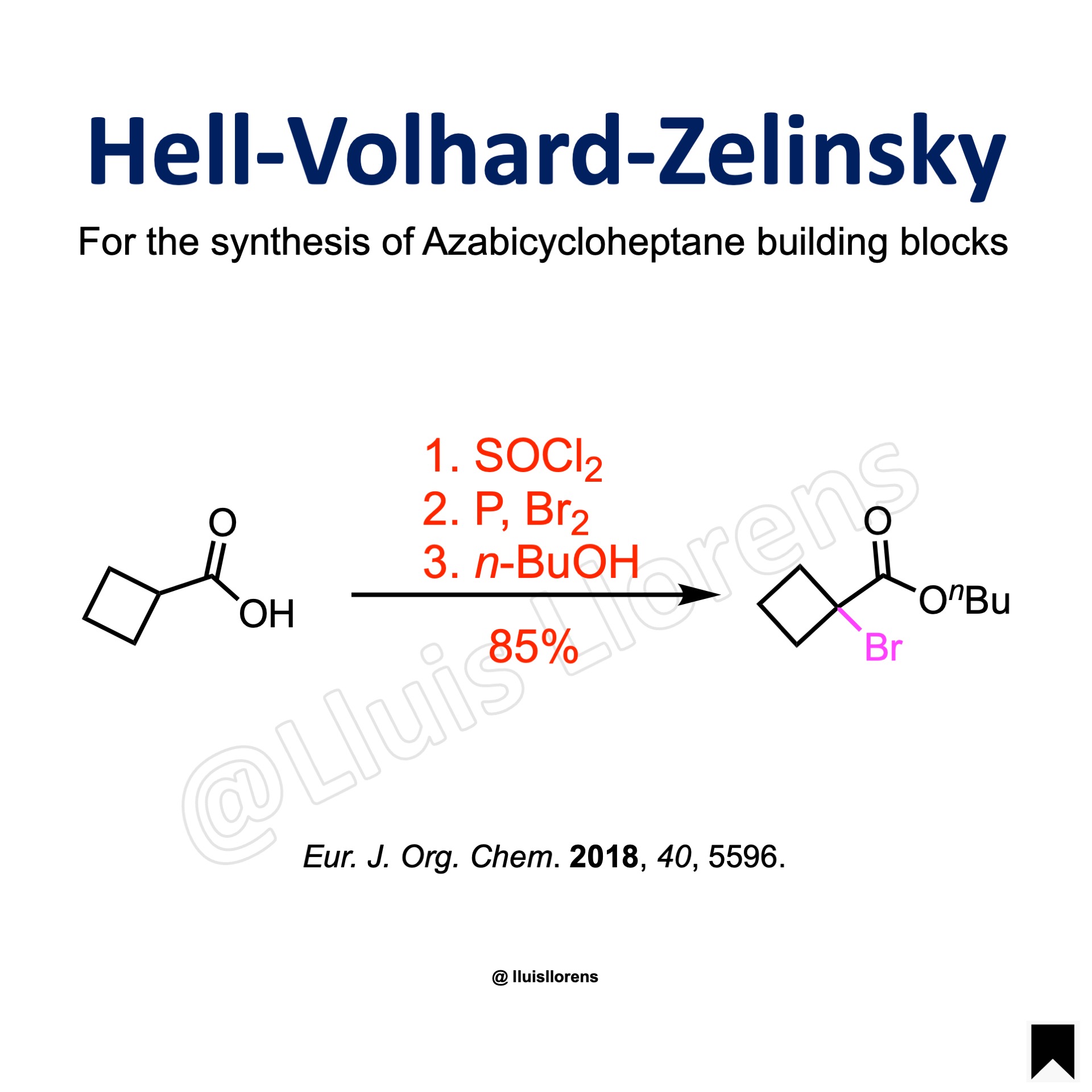
A three-necked reaction flask equipped with a thermometer and a reflux condenser was loaded with cyclobutanecarboxylic acid (1.0 mol, 1.0 eq) and SOCl2 (1.1 eq). The mixture was heated to reflux for 2 h and allowed to cool down to rt, before red phosphorus (36.1 eq) was added, followed by dropwise addition of Br2 (1.5 eq) upon heating to 50 °C. The reaction mixture was refluxed overnight, then cooled to 0 °C and poured carefully into ice-cooled n-butanol (3.0 eq) upon vigorous stirring. The resulting mixture was evaporated under reduced pressure, and the residue was distilled in vacuo to give the desired α-bromo ester (85% yield).
Learn More Named Reactions
[instagram-feed feed=2]