Grieco Elimination
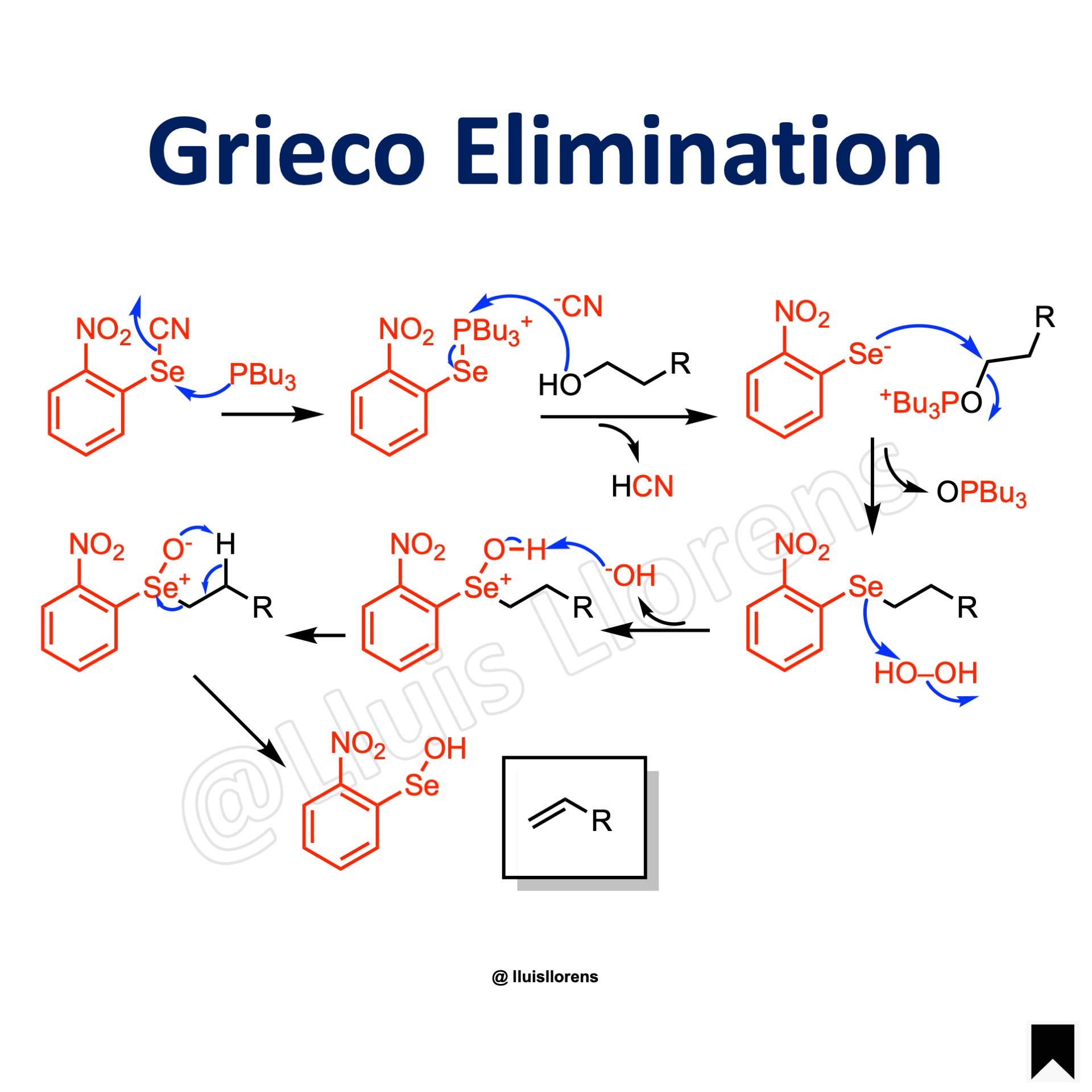
The Grieco elimination allows the dehydration of an aliphatic primary alcohol through a selenide intermediate to deliver a terminal alkene.
Reaction Mechanism
1. The phosphine displaces the cyanide. 2. Nucleophilic attack by the alcohol. 3. Formation of a selenide intermediate. 4. The selenide is oxidized to give selenoxide. 5. The selenoxide decomposes to form an alkene with expulsion of selenol.
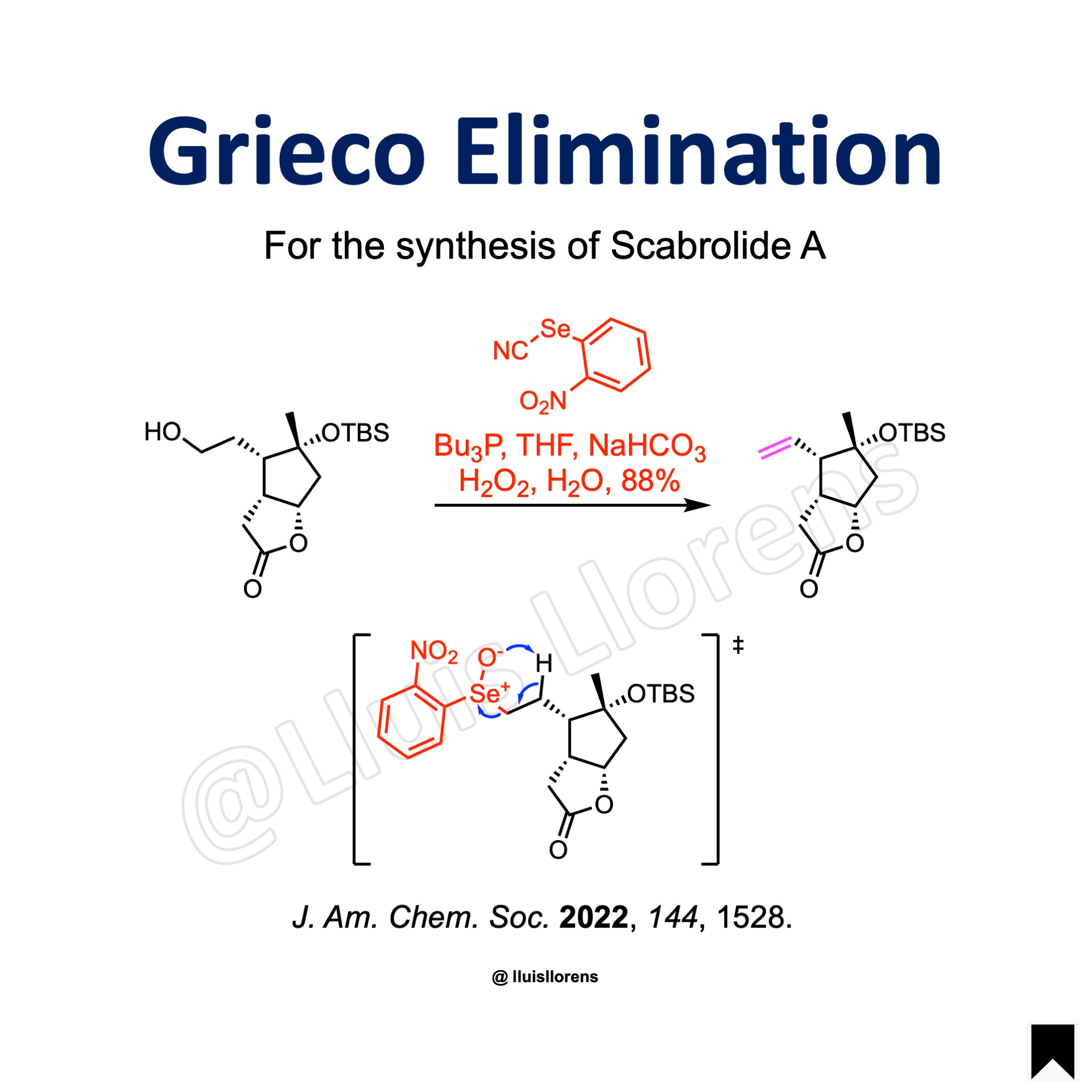
Example
J. Am. Chem. Soc. 2022, 144, 1528. (OPEN ACCESS.)
Experimental Procedure
o–Nitrophenylselenocyanate (1.44 eq) and n-Bu3P (1.48 eq) were successively added to a solution of the alcohol (7.03 mmol, 1.0 eq) in THF (46 mL) at ambient temperature, and the reaction mixture was stirred at this temperature for 3 h. Sodium bicarbonate (5.0 eq) was added at 0 °C, followed by aqueous hydrogen peroxide (35% w/w in H2O, 7.3 eq). The resulting mixture was warmed to ambient temperature and stirred for 2 h. The reaction was quenched with sat. Na2S2O3, the aqueous phase was extracted with EtOAc, the combined extracts were washed with brine and dried with MgSO4. After filtration and evaporation of the solvent, the crude product was purified by flash chromatography on silica gel to afford the title compound (88% yield).
Learn More Named Reactions
[instagram-feed feed=2]