Friedel-Crafts Reactions
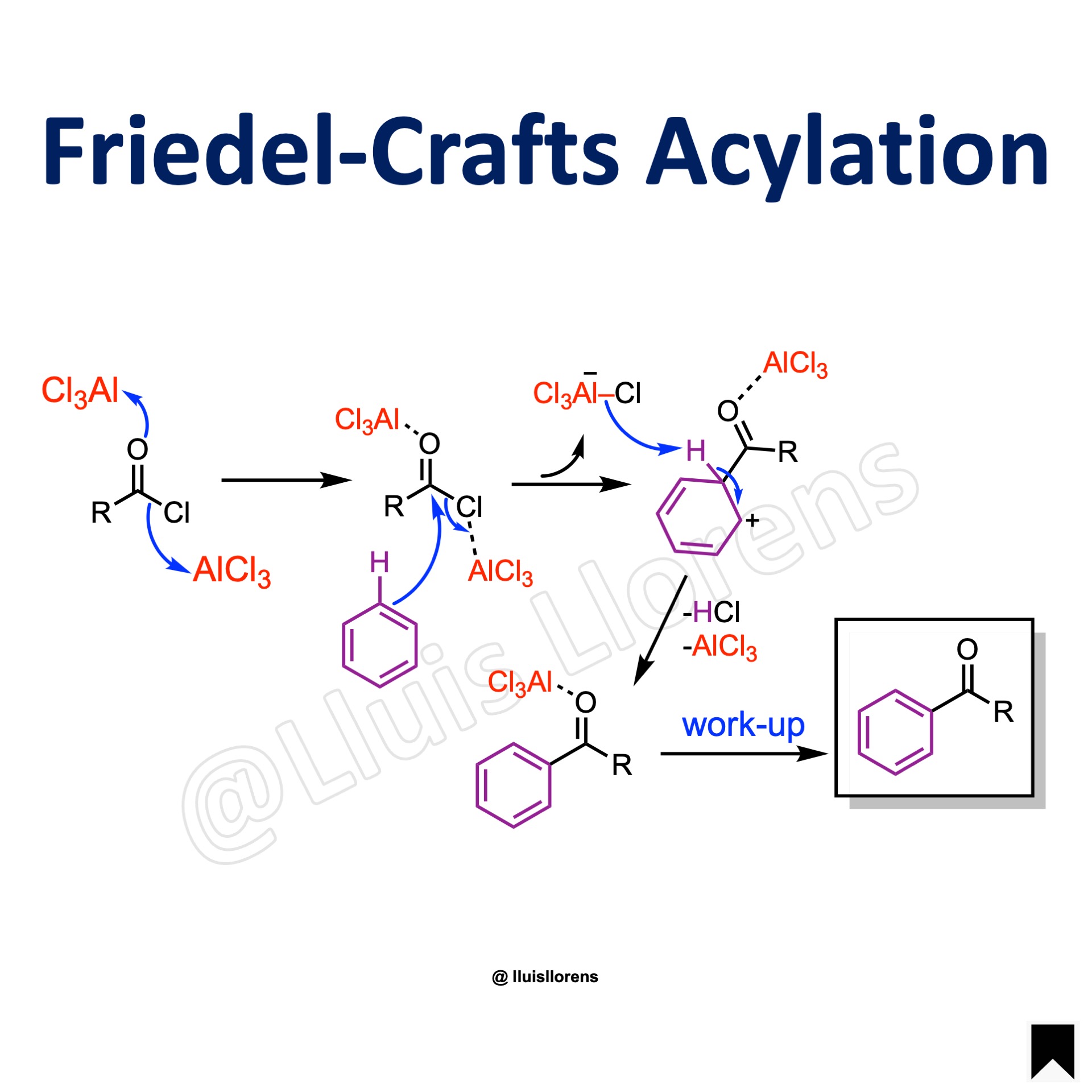
The Friedel-Crafts Acylation involves the introduction of a keto group into an aromatic or aliphatic substrate by using an acyl halide in the presence of a Lewis acid catalyst.
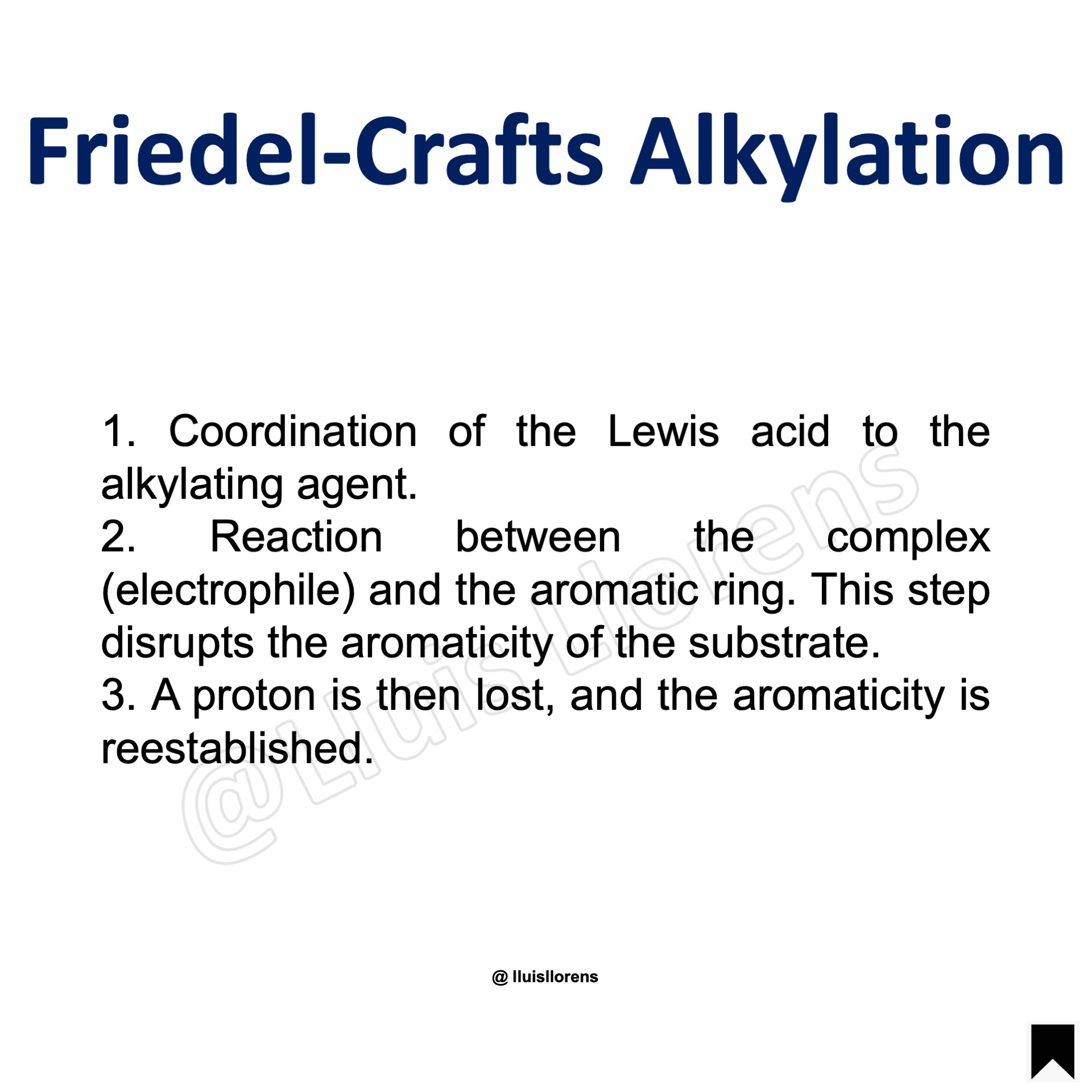
The Friedel-Crafts Alkylation involves the reaction of alkylating agents such as alkyl halides alcohols, alkenes, or alkynes in the presence of an L.A. catalyst.
General features:
Acylation: 1. Aromatic substrates with strongly electron-withdrawing groups and some heteroaromatic compounds (such as pyridines) do not undergo acylation reaction. 2. Common acylating agents are acyl halides, carboxylic acids, anhydrides, ketenes, and esters. 3. Acyl iodides are usually the most reactive. And acyl fluorides the least. 4. The intramolecular acylation has a tendency for the formation of 6-membered rings. But it is also well-suited for the closure of 5- and 7-membered rings. 5. Due to the electron-withdrawing effect of the carbonyl group, the ketone product is always less reactive than the original molecule, so multiple acylations do not occur.
Alkylation: 1. Aromatic substrates with strongly electron-withdrawing groups do not undergo alkylation reaction. 2. Alkyl fluorides are more reactive than alkyl iodides. 3. Tertiary alkyl halides are the most reactive, followed by secondary, and then primary alkyl halides. 4. 1° and 2° alkyl groups tend to rearrange and therefore product mixtures are formed. 5. The product is more nucleophilic than the reactant because alkyl groups are activators for the Friedel-Crafts reaction. Consequently, overalkylation may occur.
Reaction Mechanism
1. Coordination of the Lewis acid to the carbonyl group of the acylating agent. Then, the second equivalent of Lewis acid ionizes the initial complex. 2. SEAr reaction gives rise to an aromatic ketone-Lewis acid complex. 3. Hydrolysis affords the desired aromatic ketone.
1. Coordination of the Lewis acid to the alkylating agent. 2. Reaction between the complex (electrophile) and the aromatic ring. This step disrupts the aromaticity of the substrate. 3. A proton is then lost, and the aromaticity is reestablished.
Experimental Procedure
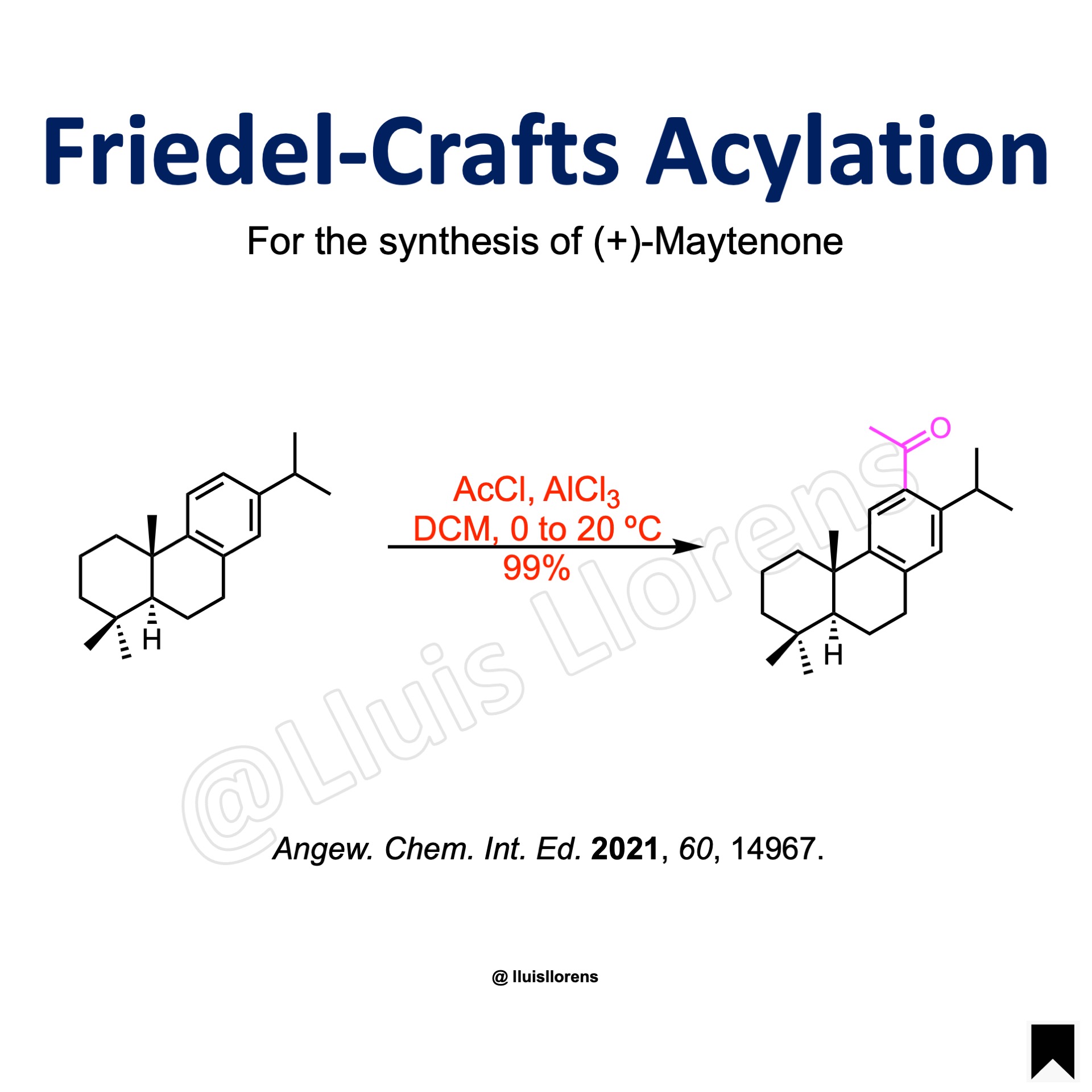
To a stirred ice-cold solution of the substrate (2.52 mmol, 1.0 eq) and acetyl chloride (4.8 eq) in dry dichloromethane (22 mL) was added AlCl3 (4.1 eq). The resulting mixture was stirred at room temperature for 2 h, after which time it was quenched by addition of 1 M aqueous HCl, and extracted with Et2O. The combined organic layers were dried over Na2SO4, filtered, and evaporated to dryness. The resulting residue was purified by column chromatography to give the acetylated product (99% yield).
Learn More Named Reactions
[instagram-feed feed=2]