Favorskii Rearrangement
The Favorskii rearrangement is the reaction between alpha-halo ketones with at least one alpha-H and a nucleophile in basic conditions to generate carboxylic acids, esters, or amides.
General features:
1. The halogen substituent can be chlorine, bromine, or iodine. 2. The base is usually an alkoxide or hydroxide. 3. Acyclic α-halo ketones give acyclic carboxylic acid derivatives. 4. Cyclic α-halo ketone substrates undergo ring-contraction reaction to afford one-carbon smaller cyclic carboxylic acids. 5. The reaction is regio- and stereoselective. 6. Alkyl or aryl substitution on the carbon bearing the halogen increases the rate of rearrangement. 7. The homo-Favorskii rearrangement takes place via a cyclobutanone intermediate when β-halo ketones are employed. 8. The quasi-Favorskii rearrangement takes place when the α-halo ketone does not have any enolizable hydrogens.
Reaction Mechanism
1. Deprotonation and formation of the enolate ion. 2. Cyclization of the enolate generates a cyclopropane intermediate. 3. Nucleophilic attack by the nucleophile. 4. Protonation leads to the ring-contracted product.
Example
Experimental Procedure
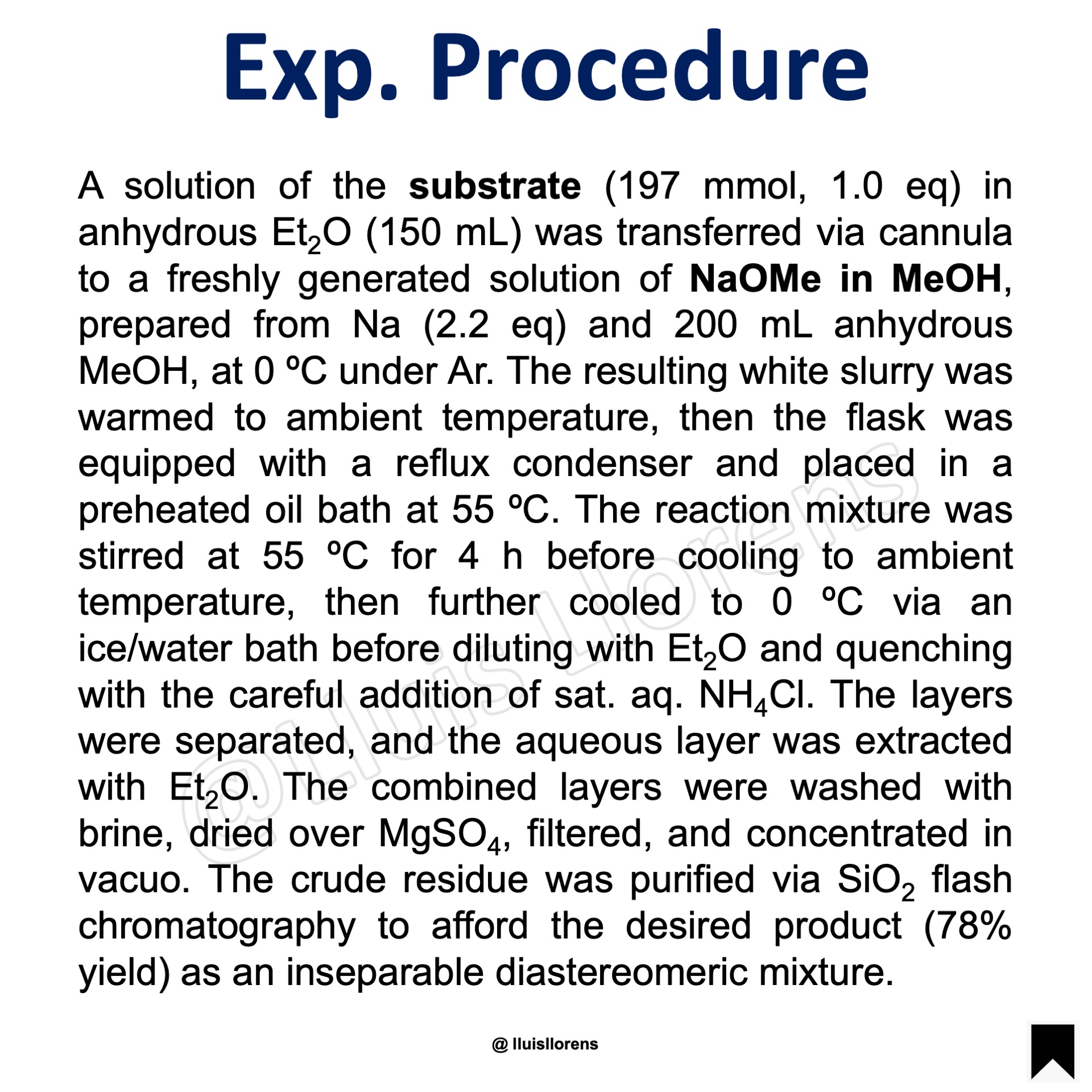
A solution of the substrate (197 mmol, 1.0 eq) in anhydrous Et2O (150 mL) was transferred via cannula to a freshly generated solution of NaOMe in MeOH, prepared from Na (2.2 eq) and 200 mL anhydrous MeOH, at 0 ºC under Ar. The resulting white slurry was warmed to ambient temperature, then the flask was equipped with a reflux condenser and placed in a preheated oil bath at 55 ºC. The reaction mixture was stirred at 55 ºC for 4 h before cooling to ambient temperature, then further cooled to 0 ºC via an ice/water bath before diluting with Et2O and quenching with the careful addition of sat. aq. NH4Cl. The layers were separated, and the aqueous layer was extracted with Et2O. The combined layers were washed with brine, dried over MgSO4, filtered, and concentrated in vacuo. The crude residue was purified via SiO2 flash chromatography to afford the desired product (78% yield) as an inseparable diastereomeric mixture.
Learn More Named Reactions
[instagram-feed feed=2]