Eschweiler-Clarke Reaction
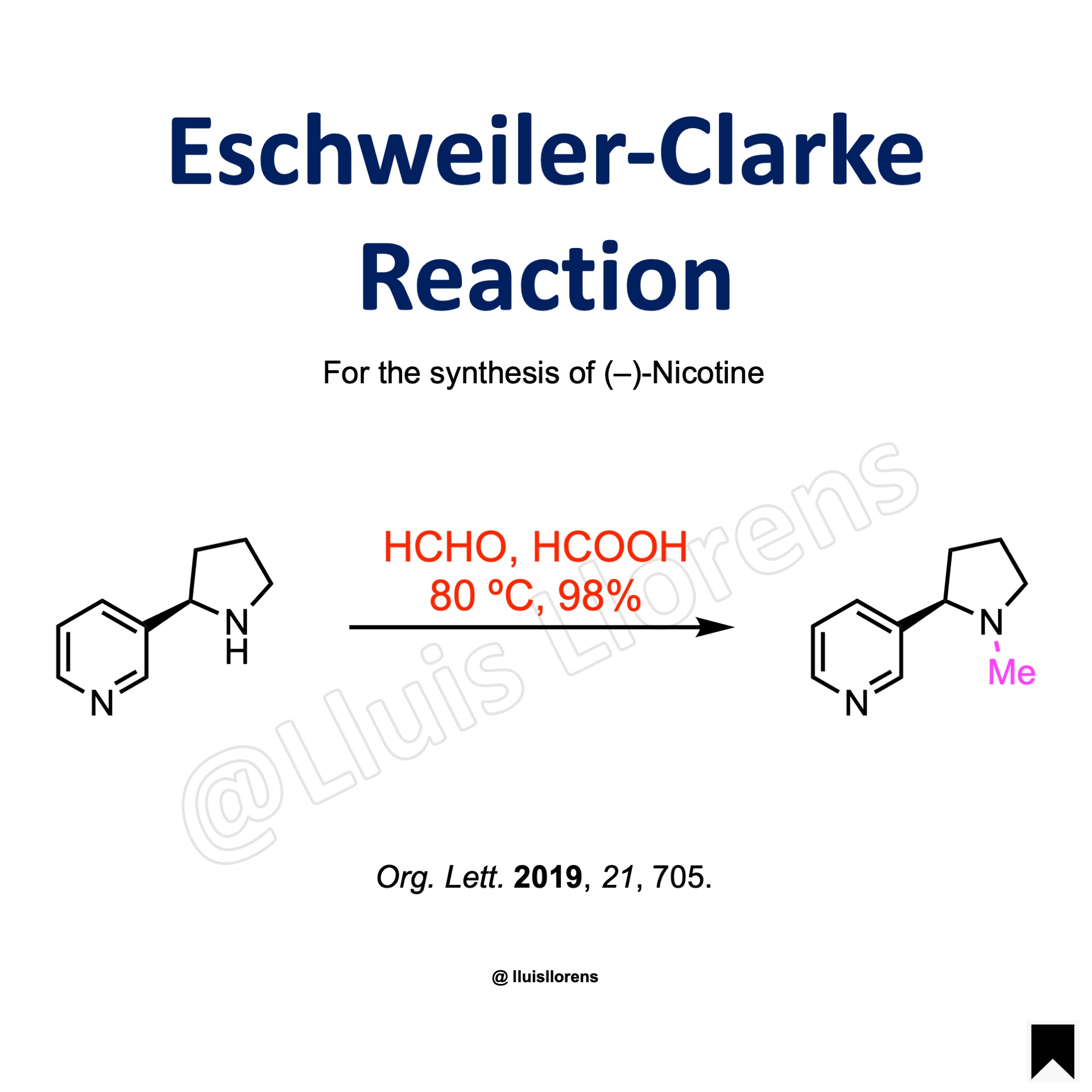
The Eschweiler-Clarke reaction allows the methylation of a primary or secondary amine to the corresponding tertiary amine, using excess formic acid and formaldehyde, avoiding the formation of quaternary ammonium salts.
Reaction Mechanism
1. Reaction between the amine and formaldehyde generates an aminoalcohol intermediate. 2. Dehydration delivers an iminium ion. 3. Hydride transfer from the reducing agent to the protonated imine delivers the methylated amine. 4. This process is repeated until a tertiary amine is generated, at which point the reaction stops.
Example
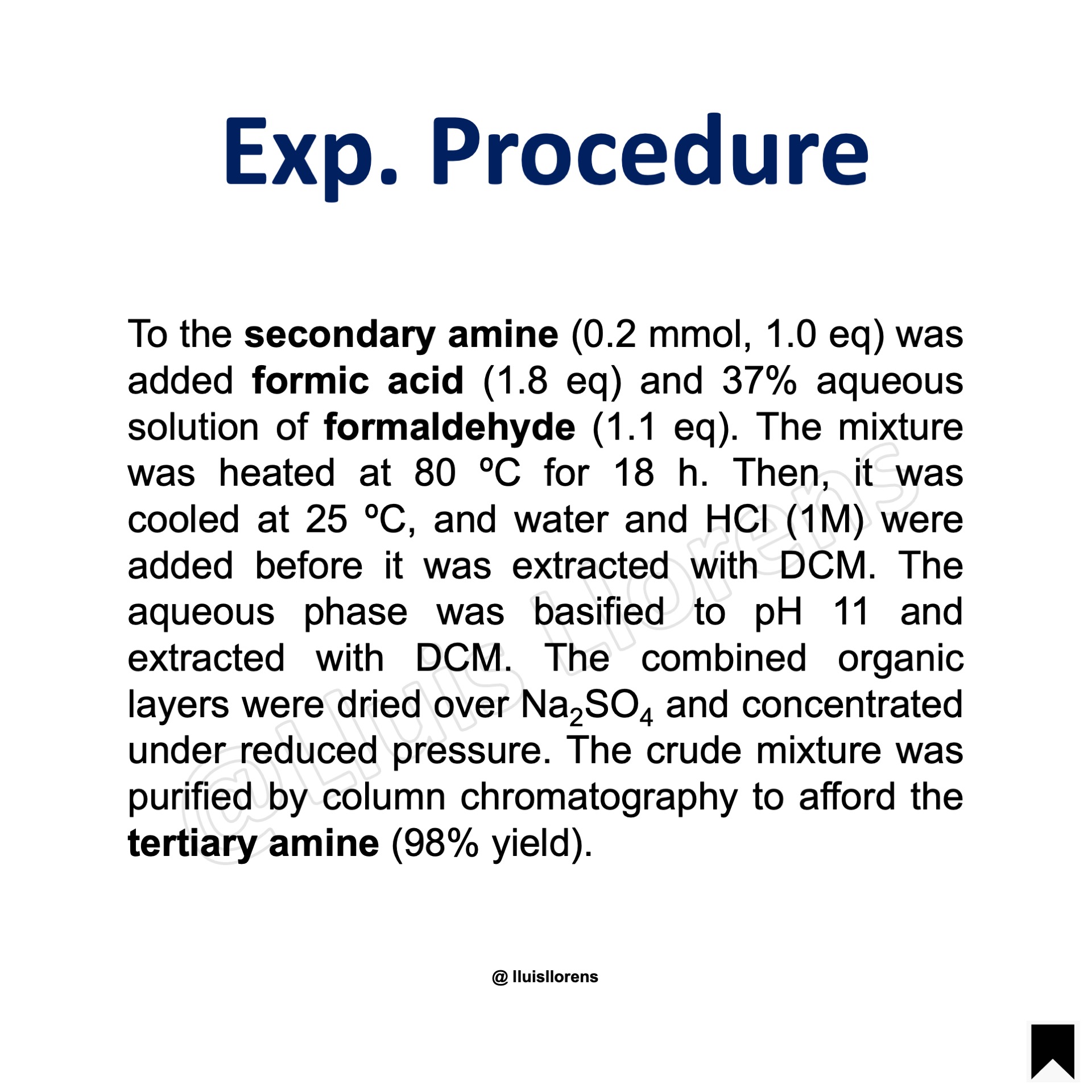
Experimental Procedure
To the secondary amine (0.2 mmol, 1.0 eq) was added formic acid (1.8 eq) and 37% aqueous solution of formaldehyde (1.1 eq). The mixture was heated at 80 ºC for 18 h. Then, it was cooled at 25 ºC, and water and HCl (1M) were added before it was extracted with DCM. The aqueous phase was basified to pH 11 and extracted with DCM. The combined organic layers were dried over Na2SO4 and concentrated under reduced pressure. The crude mixture was purified by column chromatography to afford the tertiary amine (98% yield).
Learn More Named Reactions
[instagram-feed feed=2]