Eschenmoser-Claisen Rearrangement
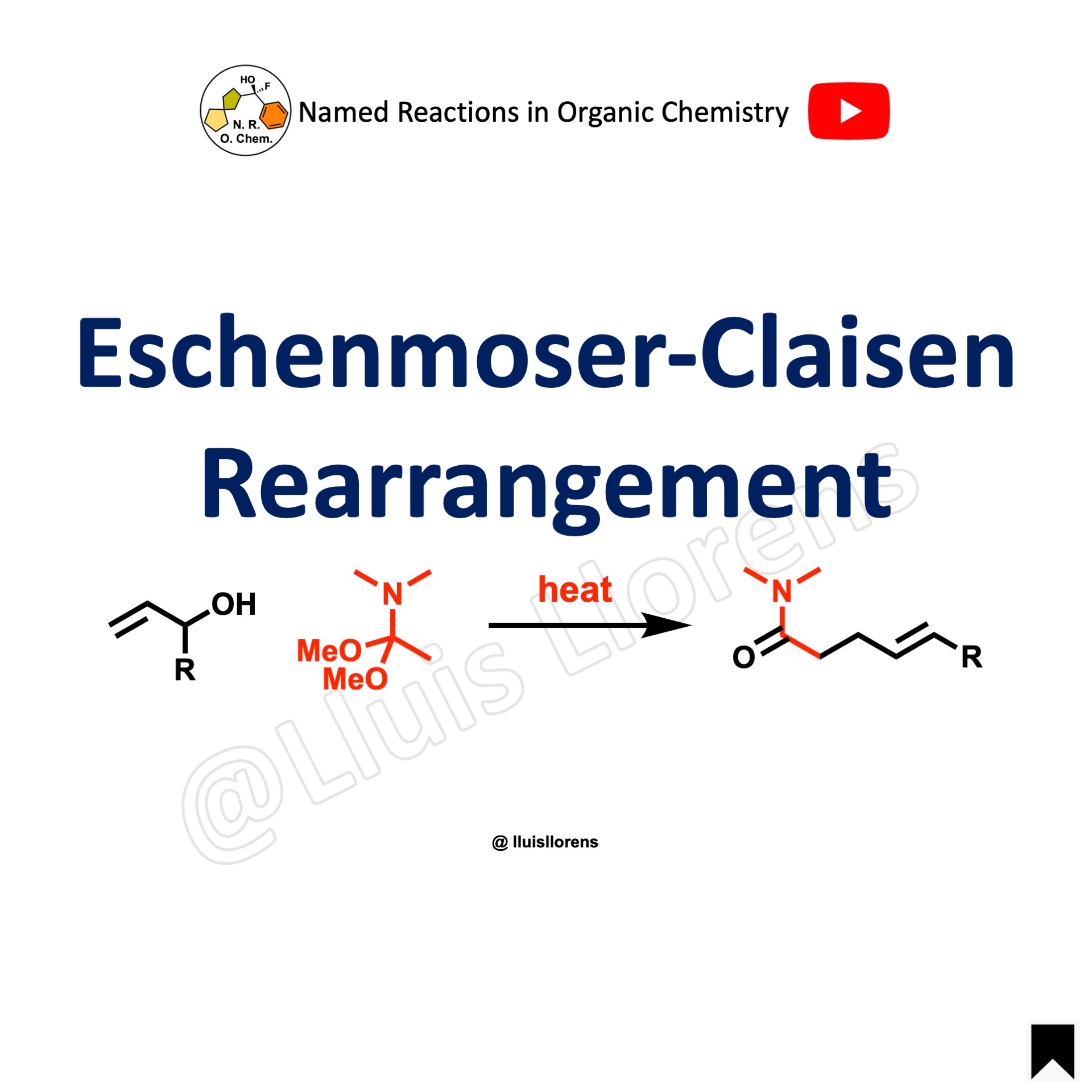
The Eschenmoser-Claisen rearrangement is the reaction in which allylic or benzylic alcohols undergo [3,3]-rearrangement when heated with N,N-dimethylacetamide dimethyl acetal to yield a gamma,delta unsaturated amide.
General features:
1. The Eschenmoser-Claisen rearrangement of secondary allylic alcohols proceeds with very high (E)-selectivity due to destabilizing 1,3-diaxial interactions in the transition state that would lead to the (Z)-isomer. 2. Allylic alcohols substituted at the 2-position afford trisubstituted alkenes.
Reaction Mechanism
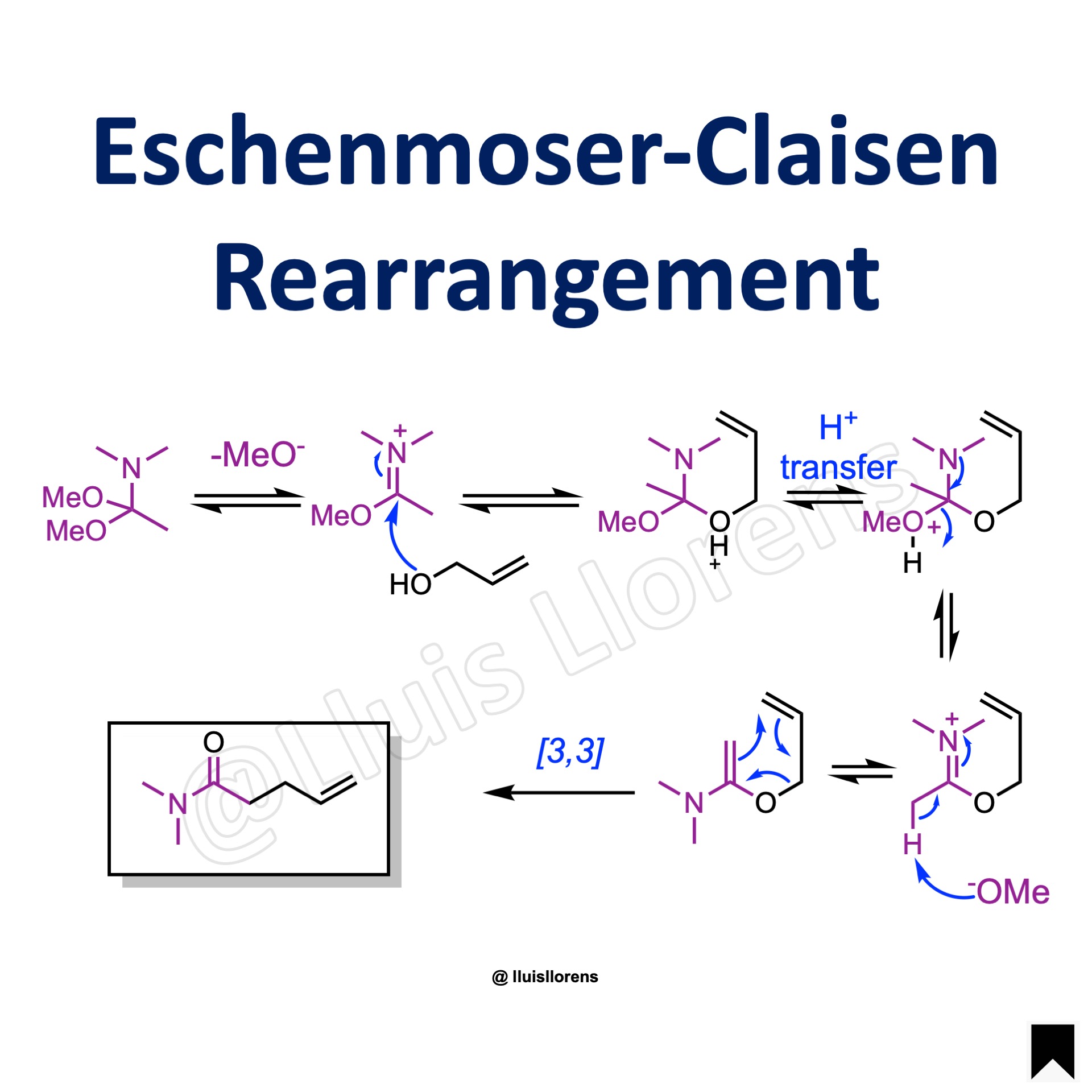
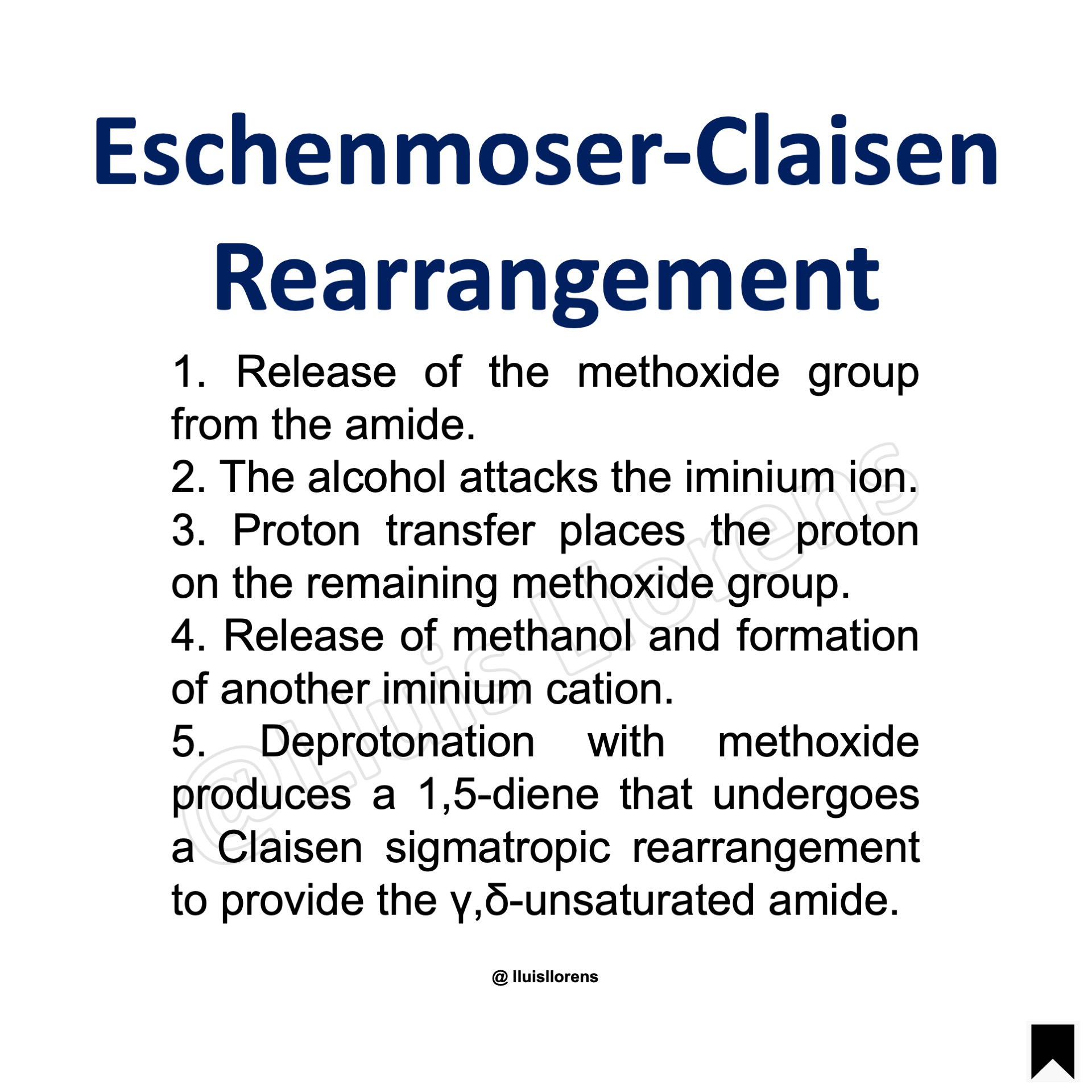
1. Release of the methoxide group from the amide. 2. The alcohol attacks the iminium ion. 3. Proton transfer places the proton on the remaining methoxide group. 4. Release of methanol and formation of another iminium cation. 5. Deprotonation with methoxide produces a 1,5-diene that undergoes a Claisen sigmatropic rearrangement to provide the γ,δ-unsaturated amide.
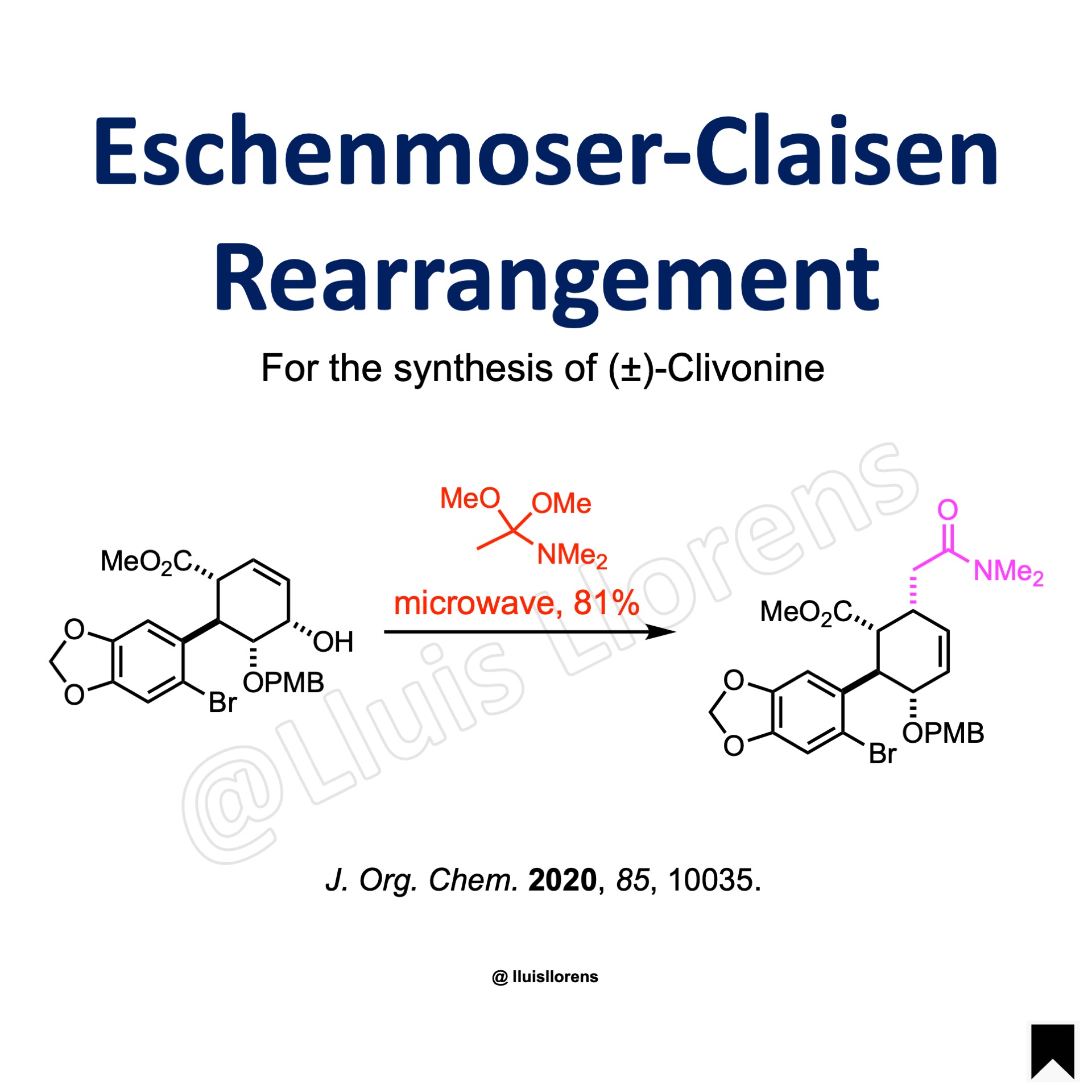
Example
Experimental Procedure
To a 10 mL sealed tube were charged the allyl alcohol (0.42 mmol, 1.0 eq), N,N-dimethylacetamide dimethyl acetal (2.15 mmol, 5.1 eq) and xylene (4 mL) at room temperature. After sealing the tube, the mixture was heated under microwave irradiation for 30 min (power = 150 W, ramp time = 5 min, ramp to temperature = 160 ºC, hold time = 25 min, stirring = high). The reaction mixture was concentrated in vacuo and purified by flash column chromatography to give the corresponding amide (81% yield).
Learn More Named Reactions
[instagram-feed feed=2]