Dieckmann Condensation
The Dieckmann condensation is the intramolecular reaction between an ester, containing an alpha-hydrogen atom, and another ester to give a beta-keto ester. Basically, an intramolecular Claisen Condensation.
General features:
1. The Dieckmann condensation generates 5-, 6-, 7-, and 8-membered rings in high yield. 2. Due to the steric stability of five- and six-membered rings, these structures will preferentially be formed. 3. 1,6 diesters will form five-membered cyclic β-keto esters. 4. 1,7 diesters will form six-membered rings.
Reaction Mechanism
1. Deprotonation of an ester at the α-position to generate an enolate ion. 2. The enolate attacks the carbonyl group of the second ester. Ring formation. 3. The tetrahedral intermediate breaks down to the enolate of the beta-keto ester. 4. Protonation of the enolate upon aqueous acidic workup affords the final product.
Example
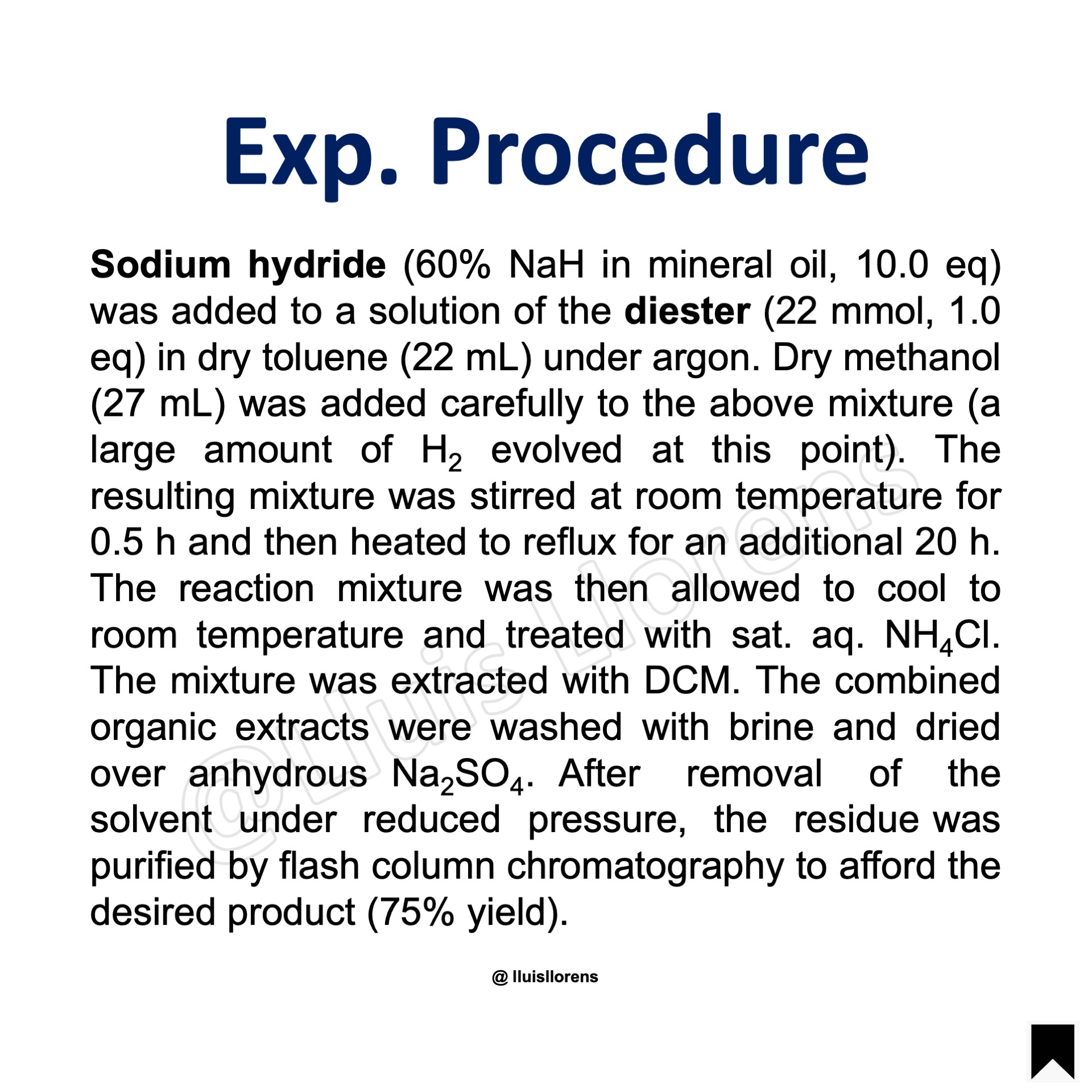
Experimental Procedure
Sodium hydride (60% NaH in mineral oil, 10.0 eq) was added to a solution of the diester (22 mmol, 1.0 eq) in dry toluene (22 mL) under argon. Dry methanol (27 mL) was added carefully to the above mixture (a large amount of H2 evolved at this point). The resulting mixture was stirred at room temperature for 0.5 h and then heated to reflux for an additional 20 h. The reaction mixture was then allowed to cool to room temperature and treated with sat. aq. NH4Cl. The mixture was extracted with DCM. The combined organic extracts were washed with brine and dried over anhydrous Na2SO4. After removal of the solvent under reduced pressure, the residue was purified by flash column chromatography to afford the desired product (75% yield).
Learn More Named Reactions
[instagram-feed feed=2]