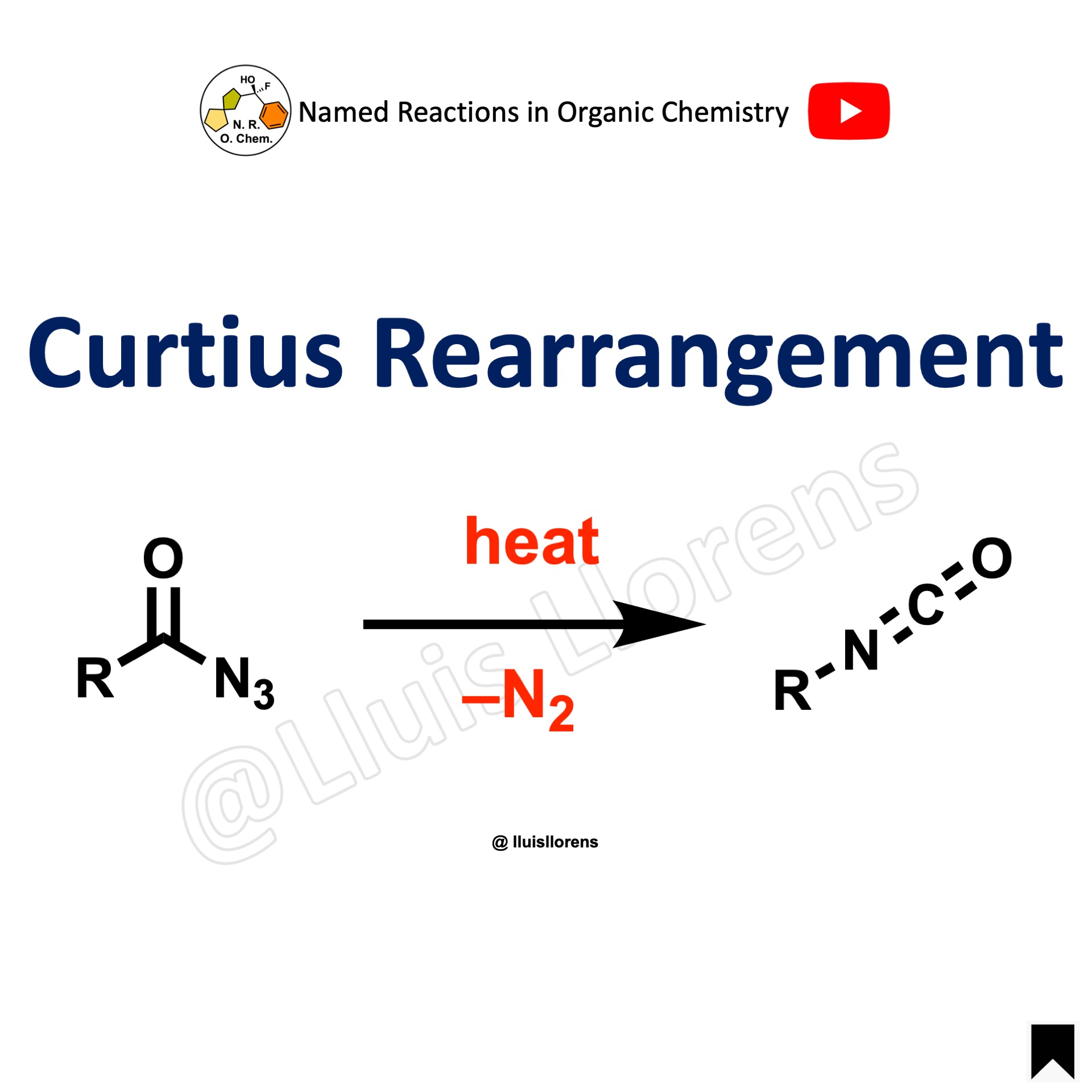
Curtius Rearrangement
The Curtius rearrangement is the thermal decomposition (pyrolysis) of acyl azides to the corresponding isocyanates.
General features:
1. The migration occurs with full retention of configuration at the R-group 2. The isocyanate can be isolated if the pyrolysis is conducted in the absence of nucleophilic solvents.
Reaction Mechanism
1. Conversion of the carboxylic acid to acid chloride or azide (by reaction with DPPA). 2. Reaction with alkali azide. 3. The acyl azide rearranges to the corresponding isocyanate. 4. The isocyanate can be isolated if the pyrolysis is conducted in the absence of nucleophilic solvents. 5. The isocyanate can undergo several reactions to deliver different products: primary amines, carbamates, or urea derivatives when the reaction is carried out in the presence of water, alcohols, or amines.
Experimental Procedure
To a stirred solution of the acid (0.33 mmol, 1.0 eq) in toluene (3.4 mL) were added Et3N (3.0 eq) and DPPA (1.5 eq) at room temperature. After stirring for 30 minutes, the mixture was heated at reflux for 2 hours. Then, the solution was cooled to room temperature. To the mixture were added allyl alcohol (10.0 eq) and DMAP (0.2 eq) at room temperature. After stirring for 14 hours at 95 °C, removal of the solvent under reduced pressure gave a crude material, which was purified by silica gel chromatography to afford the desired product (75% yield).
Learn More Named Reactions
[instagram-feed feed=2]