Cross-Coupling Reactions
This post is about cross-coupling reactions recently employed for the synthesis of natural products and drugs. You will find different reaction conditions for each of the cross-coupling reactions presented here. The post is active and growing, so you can expect more examples to be added over time. Please be sure to come back. The updates will be announced on my instagram account.
Last update 23/12/09.
A cross-coupling reaction is a type of chemical reaction in which two molecules are joined together by a covalent bond. This reaction is mediated by a metal catalyst, such as palladium or nickel. Cross-coupling reactions are important in organic synthesis because they allow for the construction of complex molecules from simple starting materials. By carefully choosing the substrates and the coupling agents, chemists can create molecules with almost any desired structure. However, these reactions are not without their challenges. One of the biggest challenges is that they often require very precise conditions in order to work correctly.
Despite these challenges, cross-coupling reactions are an essential part of synthetic organic chemistry and have led to the synthesis of many important molecules. Follow us along to find out what strategies scientists used to prepare, in some cases, even just a few milligrams of these natural products and drugs.
Buchwald-Hartwig Reactions
The Buchwald-Hartwig coupling is the Pd-catalyzed C–N or C–O bond formation between aryl halides and amines or alcohols in the presence of a stoichiometric amount of base.
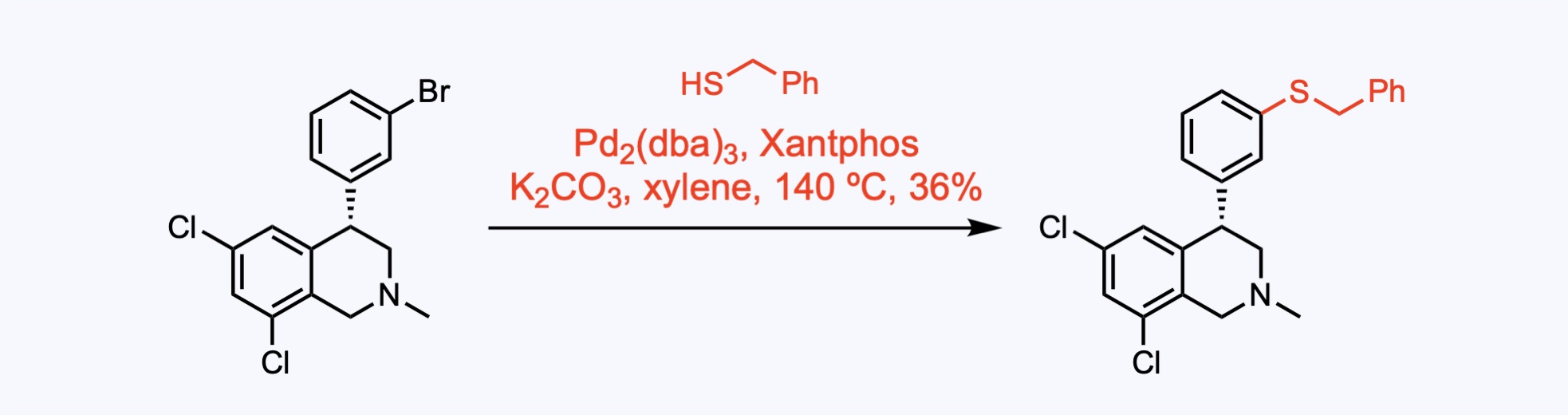
3. For the synthesis of Tenapanor hydrochloride.
Buchwald-Hartwig introduction of benzenethiol to give the mercaptan product.
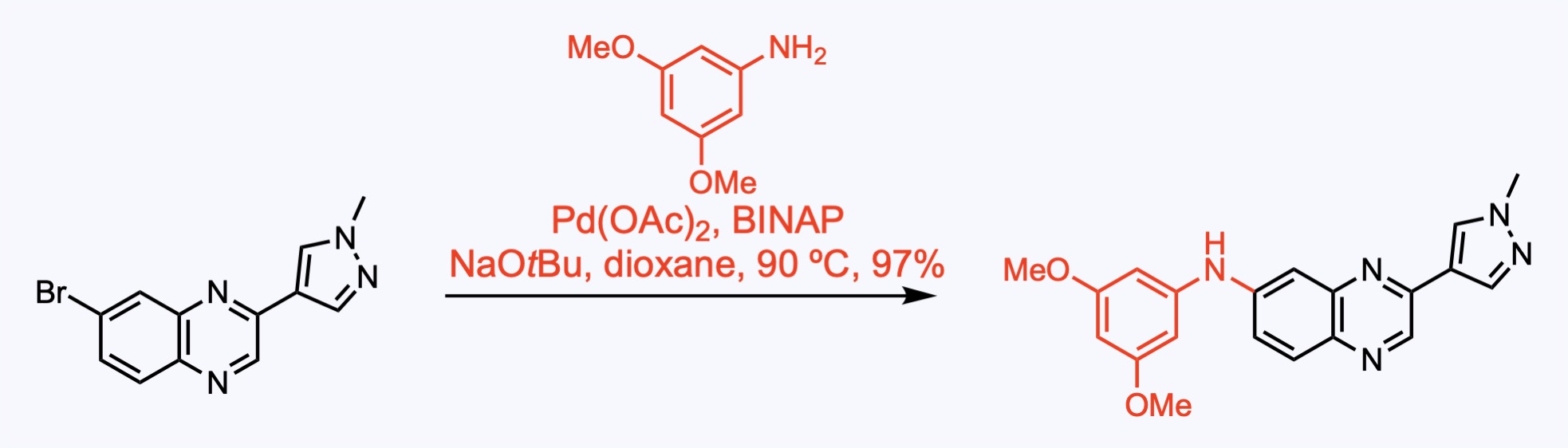
2. For the synthesis of Upadacitinib.
Palladium-catalyzed Buchwald-Hartwig amination provided the ethyl carbamate.
1. For the synthesis of Erdafitinib.
Carbonylation Reactions
Carbonylation reactions that introduce carbon monoxide into an organic substrate, delivering the corresponding carboxylic acid or ester.
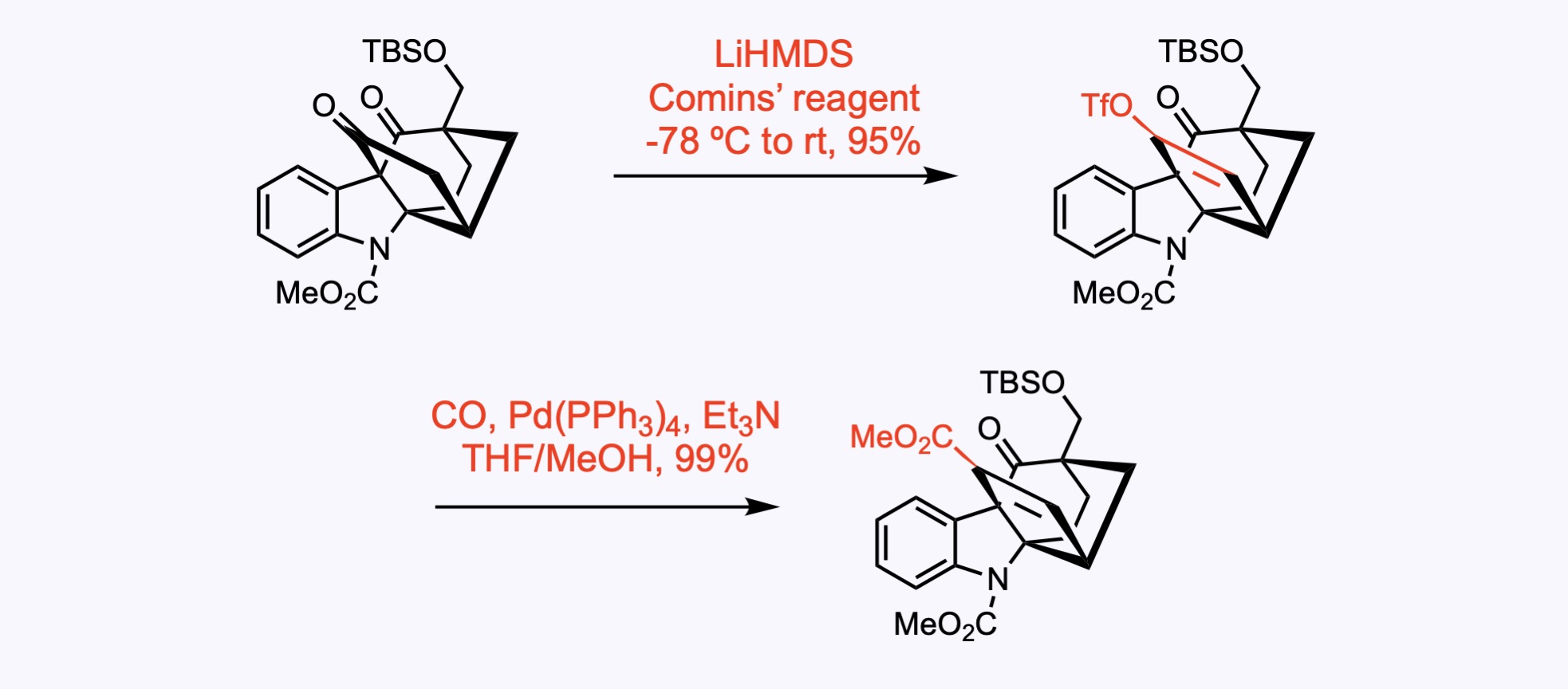
2. For the synthesis of 10,22-Dioxokopsane.
1. For the synthesis of Ubrogepant.
Chan-Lam Couplings
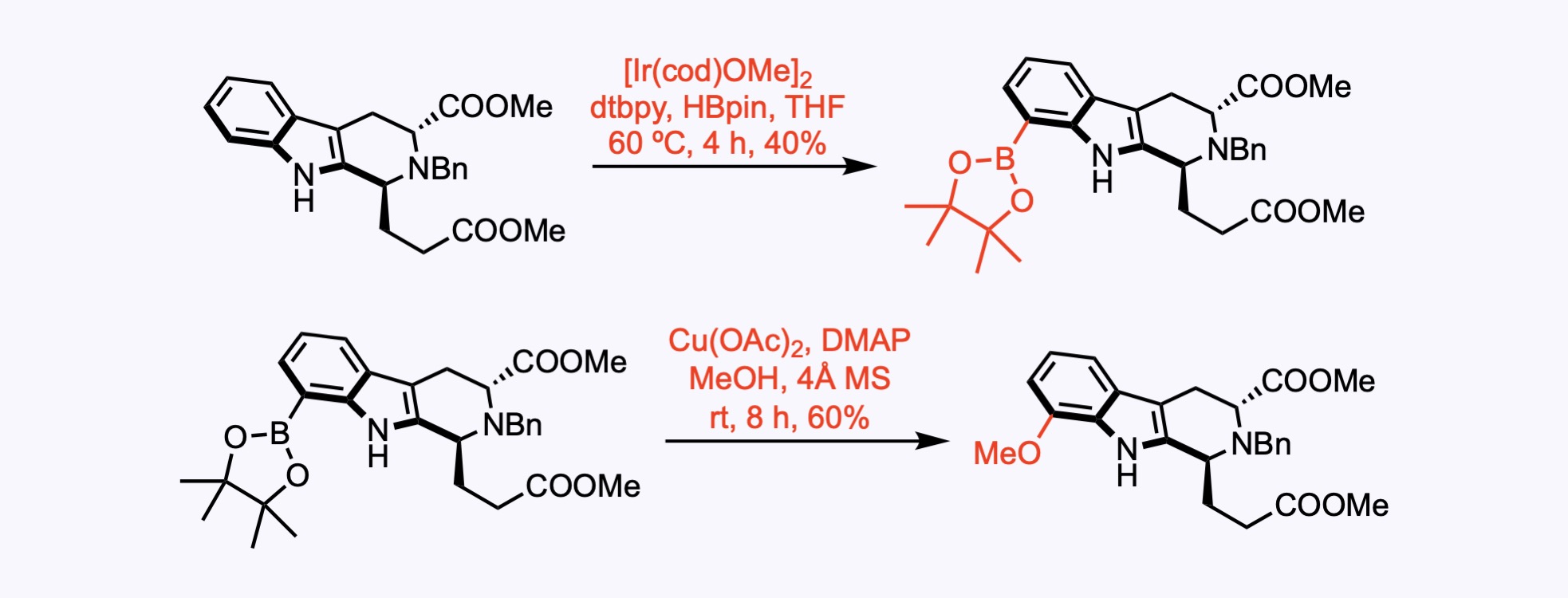
1. For the synthesis of Gardmultimine A.
A Ir-catalyzed borylation of a substituted indole. The tricyclic ester was initially borylated with high selectivity in 40% yield and then subjected to Chan−Lam coupling with methanol to afford the desired ester in 60% yield.
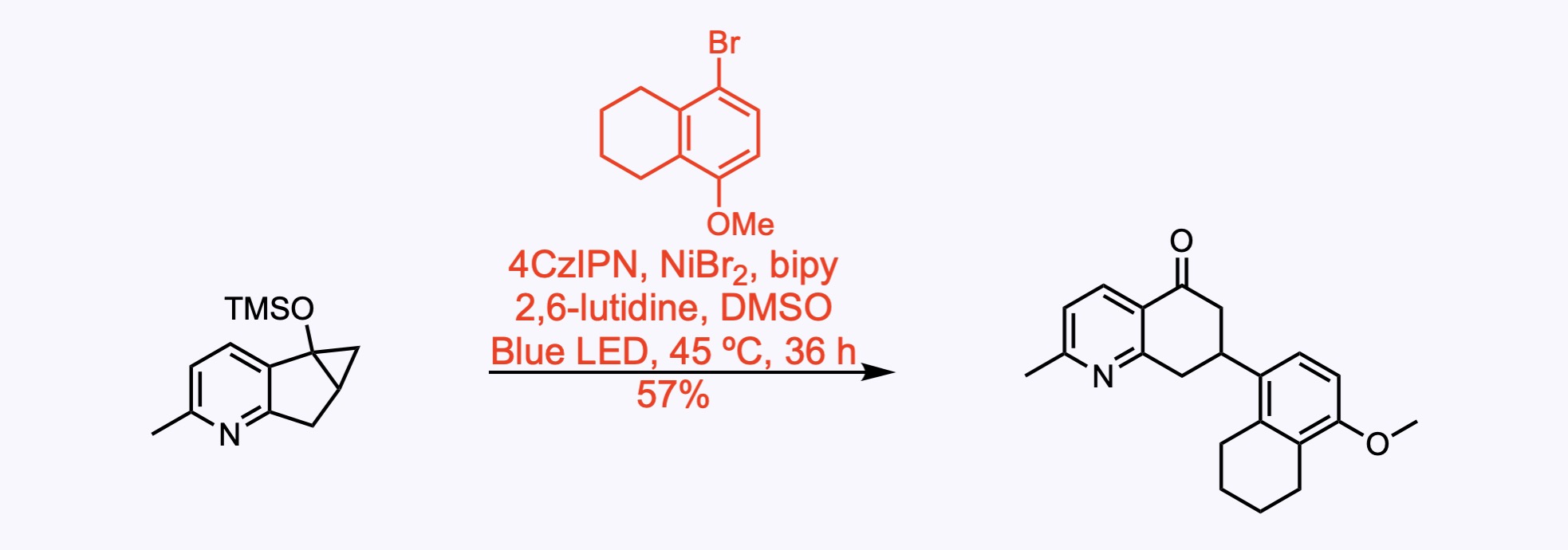
Cross-Couplings
Alternative cross-coupling reactions.
1. For the synthesis of GB22.
Radical coupling of an aryl bromide with a siloxycyclopropane, which was achieved using nickel–photoredox dual catalysis.
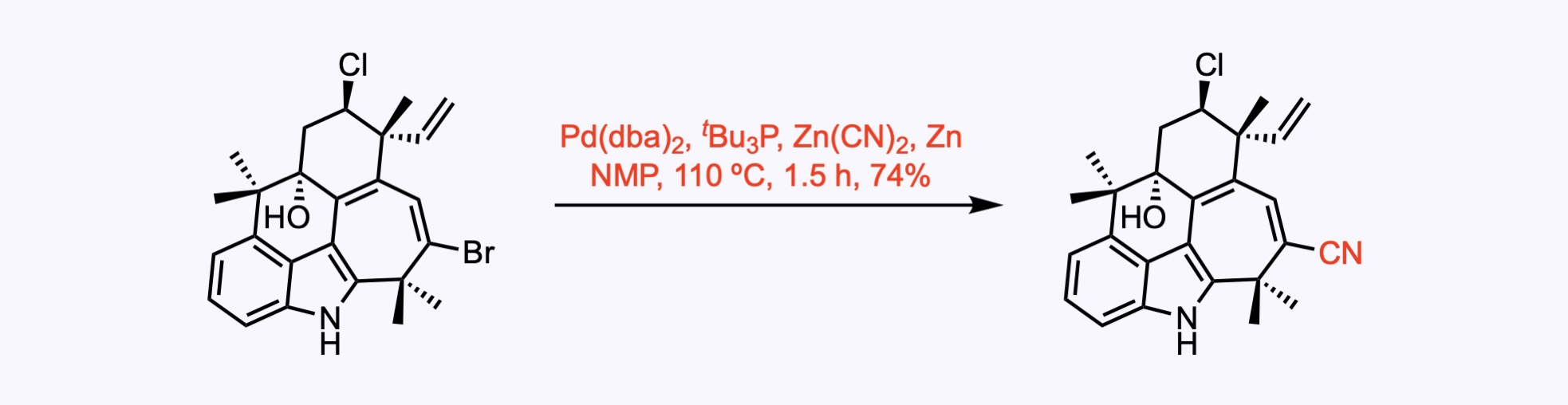
Cyanation Reactions
1. For the synthesis of Ambiguine G.
A cyanation reaction.
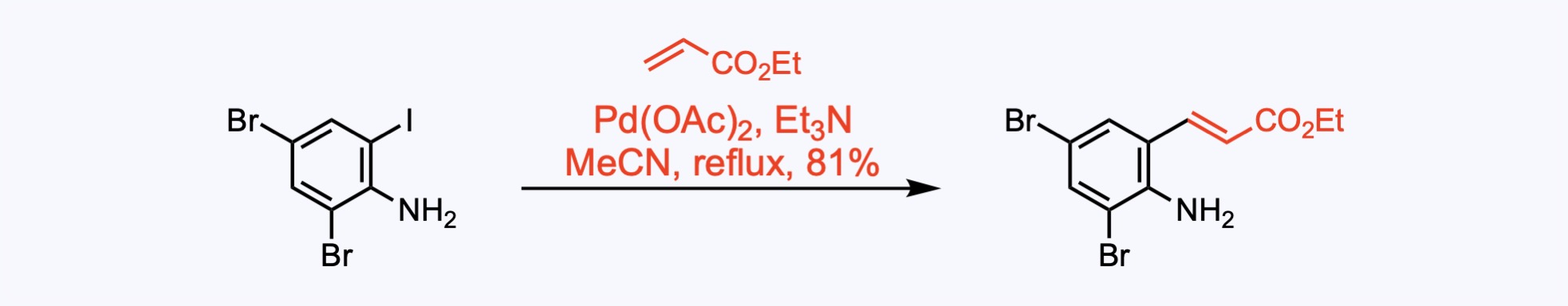
Heck Couplings
The Heck coupling allows the reaction between an unsaturated halide or triflate and an alkene in the presence of a base and a palladium catalyst.
2. For the synthesis of Hinckdentine A.
1. For the synthesis of Exatecan.
Negishi Couplings
The Negishi coupling is the nickel or palladium-catalyzed stereoselective reaction of organozincs and organic halides for the formation of carbon-carbon bonds.
1. For the synthesis of Allomatrine.
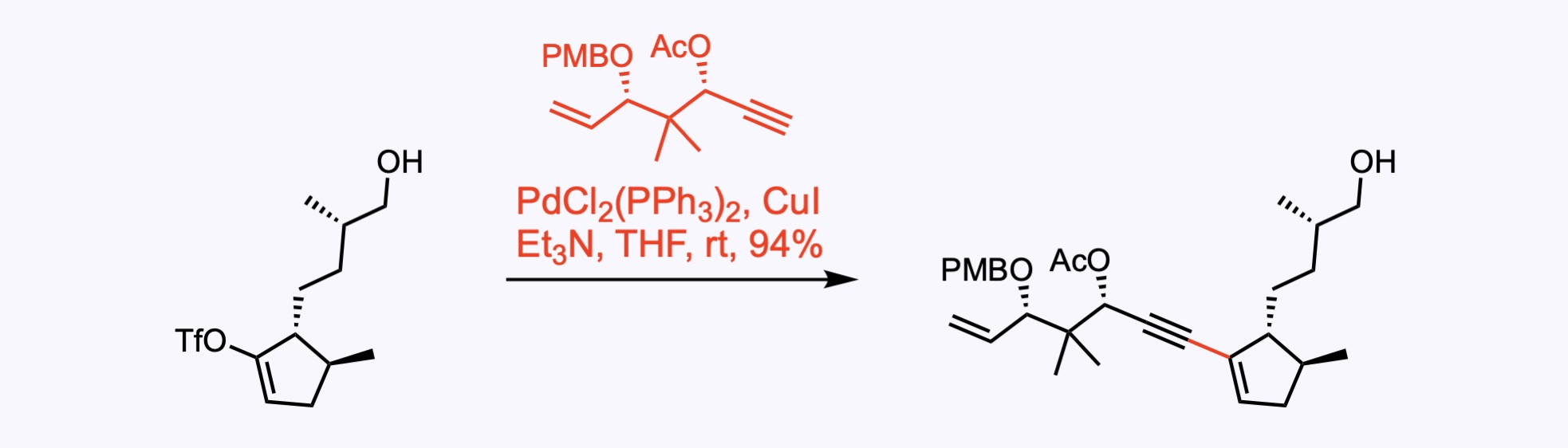
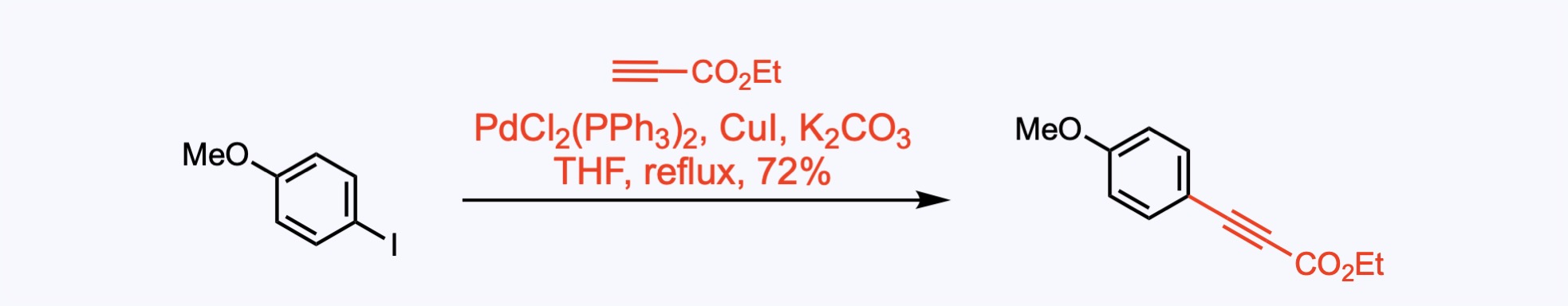
Sonogashira Couplings
The Sonogashira cross-coupling is the copper-palladium catalyzed reaction of terminal alkynes with aryl or vinyl halides.
2. For the synthesis of Aberrarone.
1. For the synthesis of Rubrolide E.
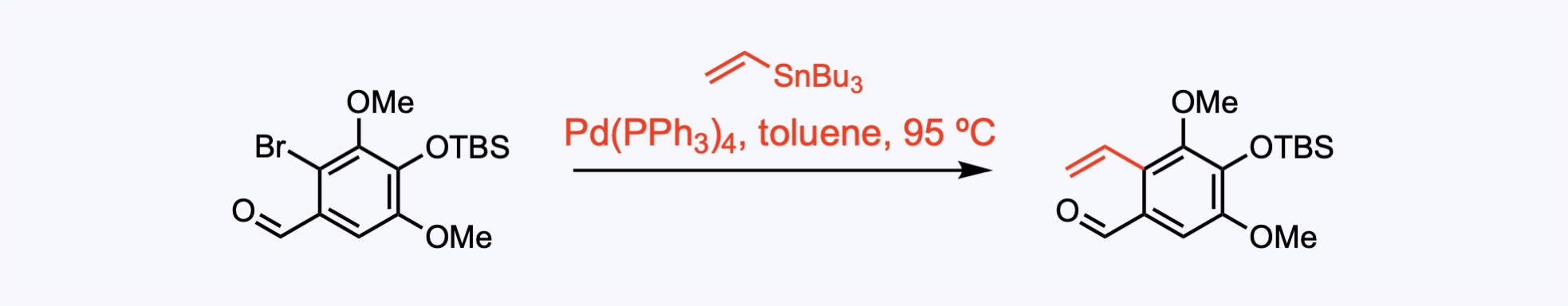
Stille Couplings
The Stille coupling is the palladium-catalyzed reaction between stannanes and halides to generate a new C–C sigma bond.
2. For the synthesis of Codonopiloneolignanin A.
Stille cross coupling to introduce the carbon−carbon double bond.
1. For the synthesis of Ambiguine G.
The vinyl triflate was prepared from the corresponding ketone with the aid of the Comins’ reagent, a triflyl-donating reagent.
J. Am. Chem. Soc. 2021, 143, 10872. Open access.
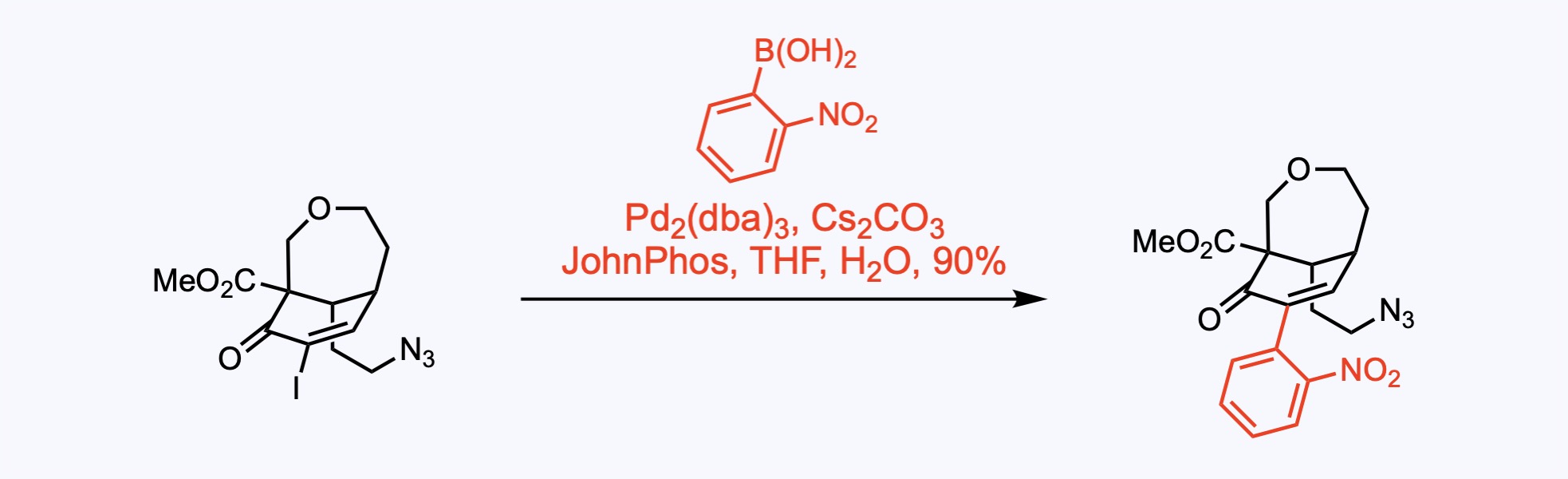
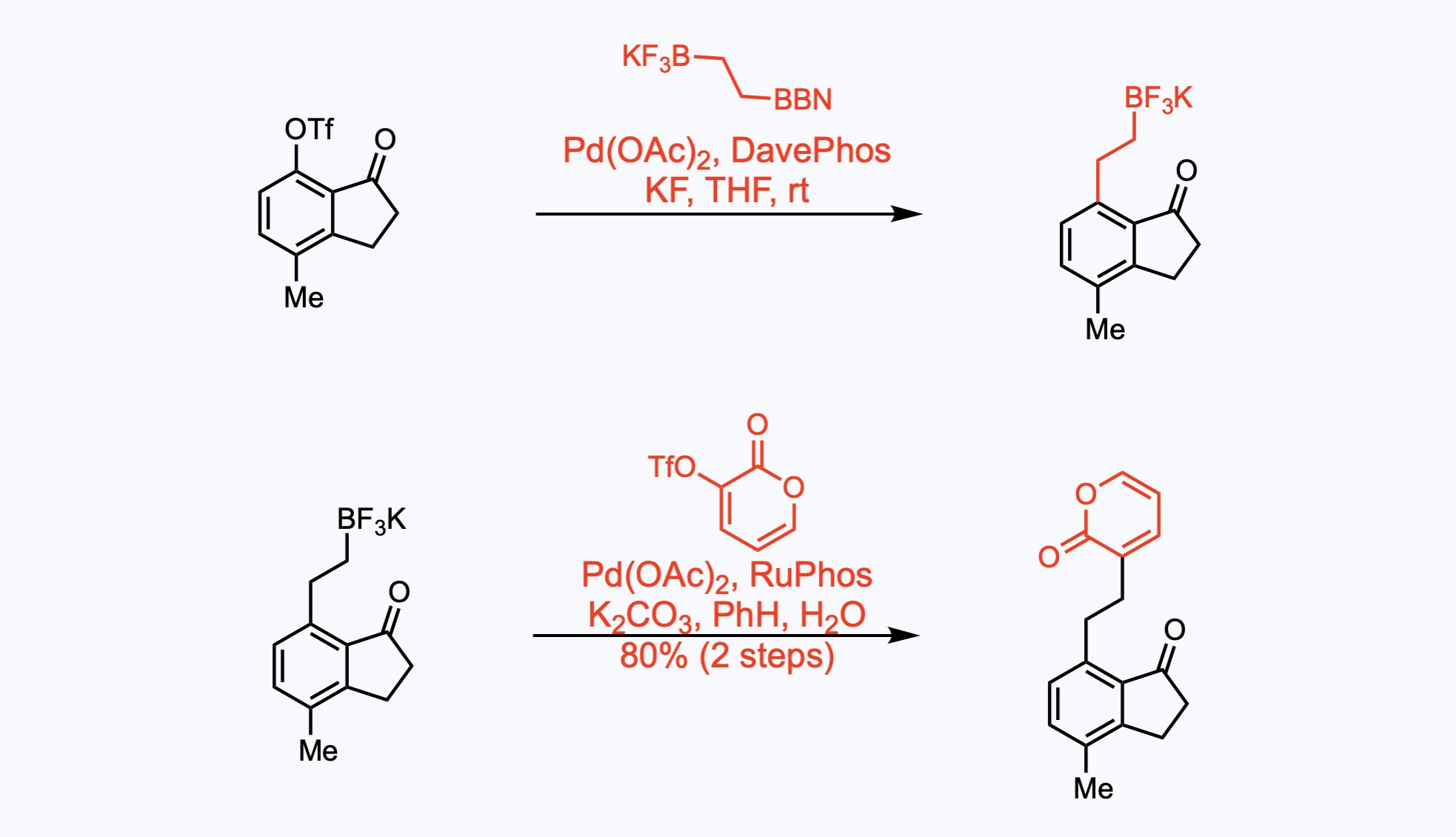
Suzuki Couplings
The Suzuki coupling is a palladium catalyzed reaction between organoboronic acids or esters and organic halides or triflates under basic conditions.
6. For the synthesis of Undulifoline.
5. For the synthesis of Cephanolide A.
The indanone and the pyrone components were successfully linked with a two-carbon fragment by an iterative cross-coupling sequence. The sp2-sp3 Suzuki cross-coupling reactions took place with the depicted organoborane, which was generated in situ from hydroboration of potassium vinyltrifluoroborate, vinylBF3K, with 9-BBN in THF at rt for 3 h. (The resulting mixture was transferred to the coupling reaction via cannula.)
4. For the synthesis of Dalesconol A.
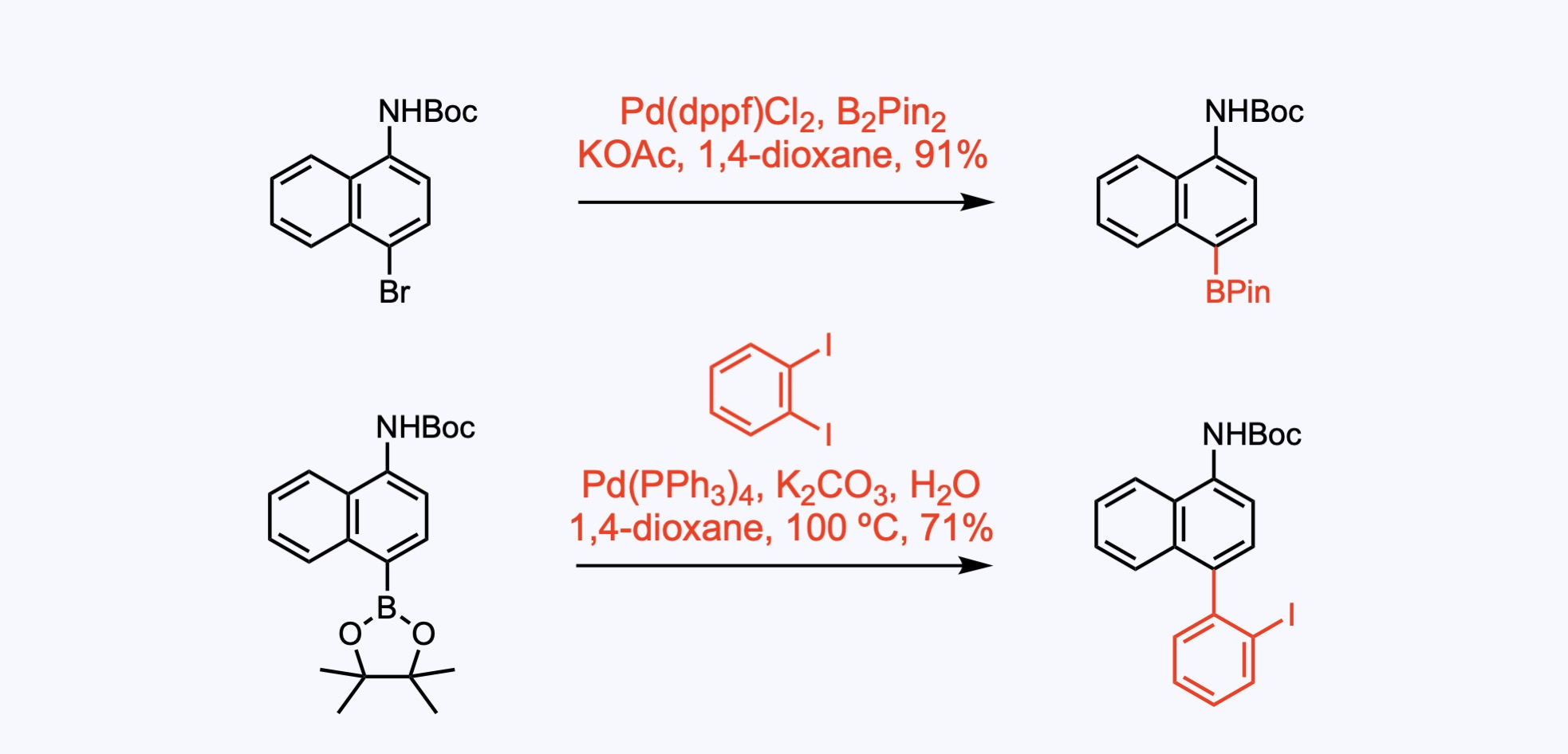
The Suzuki coupling was preceded by the Miyaura borylation reaction, which enables the synthesis of boronates by cross-coupling of bis(pinacolato)diboron, B2pin2, with the aryl bromide.
3. For the synthesis of Trifarotene.
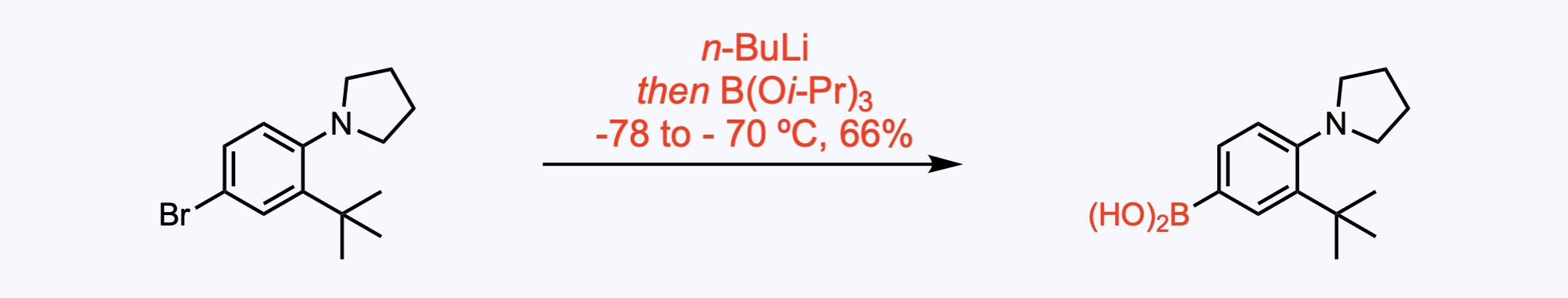
In this example, the boronic acid was generated via Li-halogen exchange and quench with triisopropylborate.
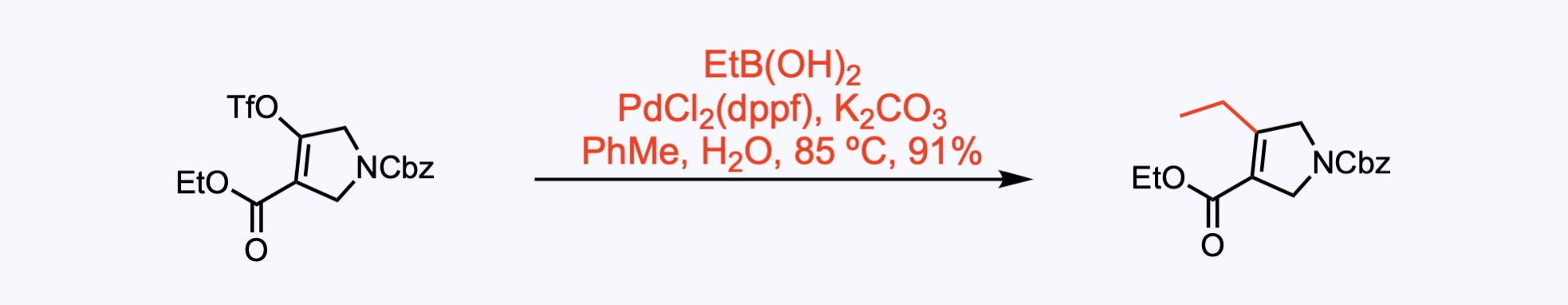
2. For the synthesis of Upadacitinib.
Suzuki-Miyaura reaction with ethylboronic acid provided 4-ethylpyrrolidine.
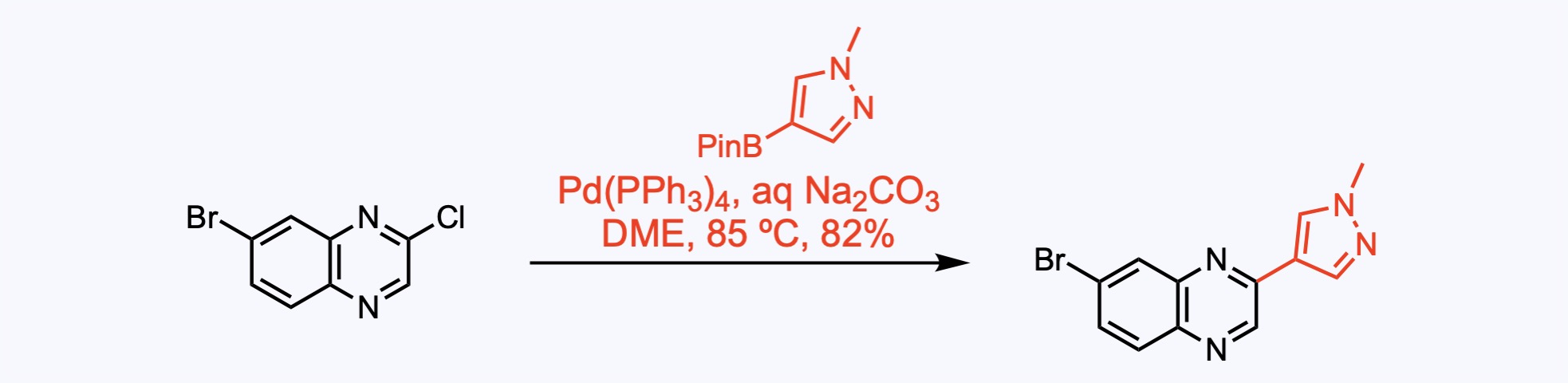
1. For the synthesis of Erdafitinib.
Suzuki coupling between the aryl chloride and the pyrazole delivered the corresponding aryl bromide, giving way to another cross-coupling reaction.
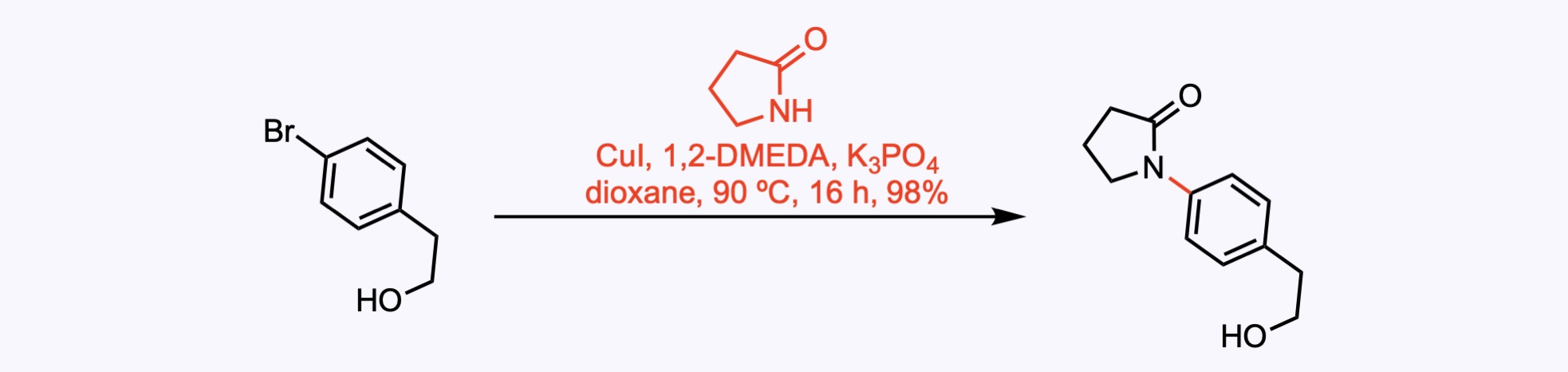
Ullmann Couplings
1. For the synthesis of Incargranine A.
A Ullmann coupling between an aryl bromide and pyrrolidinone using catalytic copper iodide and 1,2-dimethylethylenediamine.
Angew. Chem. Int. Ed. 2023, e202314308. Open access.







0 Comments